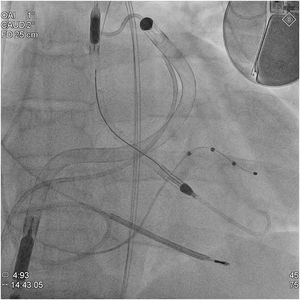
Refractory cardiogenic shock in patients with severe biventricular dysfunction is a therapeutic challenge, as it often requires short-term mechanical circulatory support (ST-MCS) devices as a bridge to heart transplant (HT). Choosing the type of ST-MCS to use is a complex process. Univentricular ST-MCS devices enhance antegrade flow, leading to increased contralateral venous return, which can cause dysfunction of the ventricle due to lack of contractile reserve. Therefore, ideally these patients benefit from biventricular ST-MCS or extracorporeal life support systems such as the venoarterial extracorporeal membrane oxygenator (VA-ECMO), which performs the function of both ventricles simultaneously. However, these devices complicate transplant surgery and clinical course. Until now, central biventricular ST-MCS has been used most commonly. The cannulas are placed directly into the heart chambers or great vessels, which means that further surgery for heart transplant can prolong graft ischemia time and encourage bleeding. Moreover, VA-ECMO is usually inserted percutaneously into central vessels and, therefore, is less invasive but entails risks, such as increased afterload of the left ventricle (LV), complications related to vascular accesses (eg, limb ischemia), and high red blood cell and platelet destruction, which must be replaced with transfusions, thereby promoting cytotoxic antibody production. The recent development of percutaneous ST-MCS for the right ventricle (RV) combined with percutaneous ST-MCS for the LV offers an alternative. We describe the first case in Spain of percutaneous implantation of the biventricular Impella (Bi-Pella) as a bridge to HT, using the Impella CP and Impella RP catheters (Abiomed Inc, United States) to partially carry out LV and RV function, respectively.
A 47-year-old man with familial dilated cardiomyopathy, severe biventricular dysfunction, and severe mitral and tricuspid regurgitation listed for HT was admitted to the Acute Cardiology Care Unit for cardiogenic shock, systemic congestion, and stage3 acute kidney injury (AKIN). Due to poor tolerance of dobutamine therapy (sustained ventricular tachycardia) and progressive impairment of kidney function (INTERMACS2), ST-MCS was considered necessary. In the preliminary assessment, echocardiographic predictors (tricuspid annular plane systolic excursion [TAPSE], 10mm; S’, 5 cm/s; RV/LV ratio, 0.9; shortening fraction, 25%) and hemodynamic predictors (filling pressure ratio, 1.6; pulmonary artery pulsatility index, 0.8; RV stroke work index, 0.3mmHg/L/m2) indicated a high risk of RV dysfunction and, therefore, percutaneous biventricular ST-MCS was performed as a bridge to HT. The devices were implanted in the catheterization lab under conscious sedation and analgesia. The ImpellaCP device was inserted through the left femoral artery (14Fr) and the ImpellaRP, through the right femoral vein (23Fr) (figure 1). Assist was initially set to levels P8 and P6, which provided flow at 3.4 and 3.1L/min, respectively. The patient gave written consent for publication of his case and the respective images in a scientific journal, with a commitment to avoid disclosing identifying information.
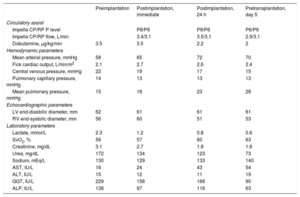
Following implantation, the patient's clinical progress was favorable (table 1), with immediate hemodynamic improvement, reduced filling volumes and pressures of both ventricles, and improved tissue perfusion and kidney function. This allowed inotropic support to be reduced with no new arrhythmic events. ST-MCS was maintained for 5days until HT, which was successful, with early extubation and withdrawal of vasoactive support (24hours) and a subsequent 11-day stay in the postoperative cardiology care unit and 30-day hospital stay. Several complications emerged during the ST-MCS period. First, initial bleeding at the venous access site (hemoglobin loss of 2.2mg/dL) in the context of thrombocytopenia (platelets, 102×103/μL) and anticoagulant therapy required local suture. Second, hemolytic anemia was treated with periodic transfusions (4units of packed red cells) and reduction of left-sided MCS to levelP6 on the fourth day, with no significant hemodynamic repercussions. Last, progressive thrombocytopenia (nadir 46×103 platelets/μL) with no related hemorrhage required a single unit of platelet concentrate to optimize hemostasis before transplantation.
Hemodynamic, echocardiographic, and laboratory parameters
| Preimplantation | Postimplantation, immediate | Postimplantation, 24 h | Pretransplantation, day 5 | |
|---|---|---|---|---|
| Circulatory assist | ||||
| Impella CP/RP P level | P8/P6 | P8/P6 | P6/P6 | |
| Impella CP/RP flow, L/min | 3.4/3.1 | 3.5/3.1 | 2.9/3.1 | |
| Dobutamine, μg/kg/min | 3.5 | 3.5 | 2.2 | 2 |
| Hemodynamic parameters | ||||
| Mean arterial pressure, mmHg | 58 | 65 | 72 | 70 |
| Fick cardiac output, L/min/m2 | 2.1 | 2.7 | 2.6 | 2.4 |
| Central venous pressure, mmHg | 22 | 19 | 17 | 15 |
| Pulmonary capillary pressure, mmHg | 14 | 13 | 13 | 13 |
| Mean pulmonary pressure, mmHg | 15 | 18 | 23 | 28 |
| Echocardiographic parameters | ||||
| LV end-diastolic diameter, mm | 62 | 61 | 61 | 61 |
| RV end-systolic diameter, mm | 56 | 60 | 51 | 53 |
| Laboratory parameters | ||||
| Lactate, mmol/L | 2.3 | 1.2 | 0.8 | 0.6 |
| SvO2, % | 56 | 57 | 60 | 63 |
| Creatinine, mg/dL | 3.1 | 2.7 | 1.8 | 1.6 |
| Urea, mg/dL | 172 | 134 | 123 | 73 |
| Sodium, mEq/L | 130 | 129 | 133 | 140 |
| AST, IU/L | 18 | 24 | 43 | 54 |
| ALT, IU/L | 15 | 12 | 11 | 19 |
| GGT, IU/L | 229 | 158 | 166 | 90 |
| ALP, IU/L | 138 | 97 | 116 | 63 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; LV, left ventricle; RV, right ventricle; SvO2, mixed venous oxygen saturation.
Three aspects of the treatment were more complex than with the univentricular Impella device. The first was coagulation control: unfractionated heparin was administered to obtain an activated clotting time (ACT) of 160 to 180s. All anticoagulation received by the patient was provided by assist purge flows plus supplemental systemic heparin to achieve this target. The purge fluids were set to 10IU/mL, for amounts of 110IU/h through the ImpellaCP (flow rate, 11 mL/h) and 140IU/h through the ImpellaRP (flow rate, 14mL/h). Supplemental unfractionated heparin was started when the ACT dropped from 250s. To prevent excessively high doses (which could cause anemia, thrombocytopenia, access-site bleeding, and kidney failure), the low setting (10IU/kg/h) was used first, ie, 600IU/h for this patient (60kg). Because the devices supplied 250IU/h, a dose of 350IU/h was prescribed at the beginning and then adjusted to the ACT test results. The second complex issue encountered was the need for invasive hemodynamic monitoring: the Swan?Ganz catheter made it possible to optimize filling pressures. Cardiac output measurement by thermodilution was not reliable because, in addition to severe tricuspid regurgitation, the ImpellaRP catheter partially bypasses the pulmonary artery and, consequently, the Fick method was used for the calculation. Based on experience with ECMO, an alternative for patients with pulsatility could be pulse waveform contour systems, which are not useful for calculating absolute cardiac output but can be used to monitor trends.1 Last, this patient exhibited higher levels of hemolysis and thrombocytopenia than usually seen.
In conclusion, this strategy offers advantages over surgical implantations and VA-ECMO,2 most notably, it is a minimally invasive procedure performed under conscious sedation and has a lower rate of vascular complications (smaller introducers), lower need for transfusions than with VA-ECMO, and lower volume needed to resuscitate the patient. However, extrapolation of these conclusions is limited, as the experience described concerns a single patient.
FUNDINGNo institution has funded this research.
AUTHORS’ CONTRIBUTIONSE. Puerto conceived this paper and drafted the manuscript. R. Martín-Asenjo collaborated in the manuscript text and performed a critical review of the text. R. Maruri, L. Domínguez-Pérez, H. Bueno, and F. Arribas-Ynsaurriaga performed a critical review of the text. All authors approved the final version of the text.
CONFLICTS OF INTERESTNone of the authors report any conflicts of interest related to the work submitted.
To Dr María Dolores García-Cosío and Dr Juan Delgado (Transplant Unit) and to Dr María Teresa Velázquez and Dr Agustín Albarrán (Interventional Cardiology) for their assistance with the patient's care.