Left ventricular noncompaction (LVNC) is characterized by multiple prominent ventricular trabeculations and deep intertrabecular recesses.1 A familial background is found in 18% to 50% of adults and the estimated prevalence in echocardiographic studies is 0.014% to 1.300%.1 Its genetic bases are heterogeneous,1–3 with only two reported mutations in the alpha-cardiac actin gene (ACTC1)3: ACTC1M271V and ACTC1E101K with additional apical hypertrophic cardiomyopathy, restrictive filling, and septal defects.4 As in other inherited familial conditions, the finding of a pathogenic mutation can be very valuable when screening at-risk relatives.5
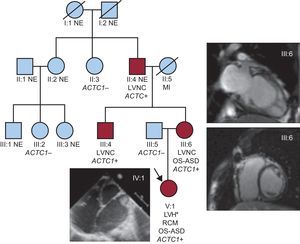
Herein we present a family with LVNC caused by the novel heterozygous ACTC1I289T mutation, which exhibited different clinical features and courses in the affected family members, namely isolated LVNC, LVNC associated with atrioseptal defect, and restrictive cardiomyopathy associated with atrioseptal defect.
A 9-month-old girl underwent heart transplantation in another hospital because of a restrictive cardiomyopathy with dilated atria, depressed left ventricular ejection fraction, and an associated small ostium secundum atrioseptal defect (proband, Figure, IV:1). At hospital discharge, the presence of a previously unsuspected LVNC was reported in the macroscopic evaluation of the heart. No histologic evaluation was carried out and no samples from the explanted heart were kept for further examination. A comprehensive workup, approved by the local research ethics committee, was offered to her first-degree relatives and the family tree was accordingly expanded. This evaluation included electrocardiogram, echocardiography, and blood sampling for genetic studies. Cardiac magnetic resonance imaging, exercise testing, and Holter-electrocardiogram were performed at cardiologist discretion. LVNC was defined following Jenni's (telesystolic noncompacted/compacted myocardium > 2 by echocardiography) and/or Petersen's criteria (telediastolic noncompacted/compacted > 2.3 by cardiac magnetic resonance imaging)1.
Familial pedigree. ACTC1, alpha-cardiac actin gene; LVNC, left ventricular noncompaction; LVH, left ventricular hypertrabeculation; MI, myocardial infarction; NE, not evaluated; OS-ASD, ostium secundum atrioseptal defect; RCM, restrictive cardiomyopathy. Circles denote females, squares males. Red symbols represent affected individuals. *Macroscopic evaluation at heart transplantation.
Sanger sequencing (MYH7, myosin binding protein C3, Nkx2.5, and ACTC1 genes) was performed in genomic DNA of the maternal proband's uncle since the proband was initially not available for mutation screening. The heterozygous ACTC1I289T mutation was identified and cascade genetic studies were undertaken in the remaining relatives. No additional mutation was found in any of the other screened genes. The Table shows the results of the family study in affected (II:4, III:4, III:6 and IV:1) and nonaffected individuals (no additional family members were available for the study).
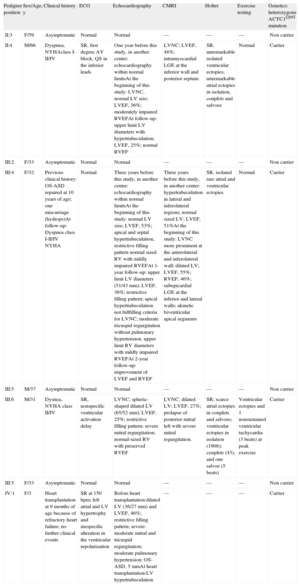
Results From the Clinical Family Evaluation
| Pedigree position | Sex/Age, y | Clinical history | ECG | Echocardiography | CMRI | Holter | Exercise testing | Genetics: heterozygous ACTC1I289T mutation |
| II:3 | F/59 | Asymptomatic | Normal | Normal | — | — | — | Non carrier |
| II:4 | M/66 | Dyspnea, NYHAclass I-II/IV | SR, first degree AV block, QS in the inferior leads | One year before this study, in another center: echocardiography within normal limitsAt the beginning of this study: LVNC, normal LV size; LVEF, 36%; moderately impaired RVEFAt follow-up: upper limit LV diameters with hypertrabeculation; LVEF, 25%; normal RVEF | LVNC; LVEF, 48%; intramyocardial LGE at the inferior wall and posterior septum | SR, unremarkable isolated ventricular ectopies, unremarkable atrial ectopies in isolation, couplets and salvoes | Normal | Carrier |
| III:2 | F/33 | Asymptomatic | Normal | Normal | — | — | — | Non carrier |
| III:4 | F/32 | Previous clinical history: OS-ASD repaired at 10 years of age; one miscarriage (hydrops)At follow-up: Dyspnea class I-II/IV NYHA | Normal | Three years before this study, in another center: echocardiography within normal limitsAt the beginning of this study: normal LV size; LVEF, 53%; apical and septal hypertrabeculation, restrictive filling pattern normal sized RV with mildly impaired RVEFAt 1-year follow-up: upper limit LV diameters (51/43 mm); LVEF, 36%; restrictive filling pattern; apical hypertrabeculation not fullfilling criteria for LVNC; moderate tricuspid regurgitation without pulmonary hypertension. upper limit RV diameters with mildly impaired RVEFAt 2-year follow-up: improvement of LVEF and RVEF | Three years before this study, in another center: hypertrabeculation in lateral and inferolateral regions; normal sized LV; LVEF, 51%At the beginning of this study: LVNC more prominent at the anterolateral and inferolateral wall; dilated LV; LVEF, 55%; RVEF, 46%; subepicardial LGE at the inferior and lateral walls; akinetic biventricular apical segments | SR, isolated rare atrial and ventricular ectopies | Normal | Carrier |
| III:5 | M/37 | Asymptomatic | Normal | Normal | — | — | — | Non carrier |
| III:6 | M/31 | Dysnea, NYHA class II/IV | SR, nonspecific ventricular activation delay | LVNC; spheric-shaped dilated LV (65/52 mm); LVEF, 25%; restrictive filling pattern; severe mitral regurgitation; normal-sized RV with preserved RVEF | LVNC; dilated LV; LVEF, 27%; prolapse of posterior mitral left with severe mitral regurgitation. | SR; scarce atrial ectopies in couplets and salvoes; ventricular ectopies in isolation (1908); couplets (43), and one salvoe (5 beats) | Ventricular ectopies and 1 nonsustained ventricular tachycardia (3 beats) at peak exercise | Carrier |
| III:5 | F/33 | Asymptomatic | Normal | Normal | — | — | — | Non carrier |
| IV:1 | F/3 | Heart transplantation at 9 months of age because of refractory heart failure; no further clinical events | SR at 150 bpm; left atrial and LV hypertrophy and inespecific alteration in the ventricular repolarization | Before heart transplantation:dilated LV (36/27 mm) and LVEF, 46%; restrictive filling pattern; severe-moderate mitral and tricuspid regurgitation; moderate pulmonary hypertension; OS-ASD, 5 mmAt heart transplantation:LV hypertrabeculation | — | — | — | Carrier |
ACTC1, alpha-cardiac actin gene; AVB: atrioventricular block; CMRI, cardiac magnetic resonance imaging; ECG, electrocardiogram; F, female; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; LVNC, left ventricular noncompaction; M, male; MI, myocardial infarction; NE, not evaluated; NYHA, New York Heart Association; OS-ASD, ostium secundum atrioseptal defect; RV, right ventricle; RVEF, right ventricular ejection fraction; SR: sinus rhythm.
Gene sequencing yielded the presence of the heterozygous ACTC1I289T mutation, not present in the National Center for Biotechnology list of single nucleotide polymorphisms in the ACTC1 gene. Although hundreds of variants have been identified in sarcomeric and desmosomal genes, only a few polymorphisms and < 30 mutations causing any kind of cardiomyopathy have been described in the ACTC1 gene, suggesting that changes in the ACTC1 gene are poorly tolerated. Actin is essential for cell morphology, adhesion, and migration. This novel variant alters a preserved amino acid residue (I289) in the protein, replacing a nonpolar (isoleucine) with another polar and noncharged (threonine) aminoacid, thus causing moderate modifications in the physicochemical properties related to the hydrophobicity, charge, polarity, and mass of the protein (Grantham distance 89 [0-215]). The prediction of in silico (SIFT [Sorting Intolerant from Tolerant], Polyphen-2, and Pmut) analyses neither confirmed nor ruled out its pathogenity (inconclusive results with low confidence). The preserved I289 amino acid residue maps to subdomain 3, important for the stability and polymerization of the actin filaments6 and next to the myosin binding site, possibly disrupted by the presence of the ACTC1I289T mutation. Furthermore, our ACTC1I289T mutation cosegregated perfectly with the LVNC phenotype, with a 100% penetrance in the individuals available for the study.
We acknowledge that a more thorough genetic study could have included many other genes. Nonetheless, we considered it finished in terms of cost-effectiveness for three reasons: a) our results were consistent with a previous study linking LVNC and septal defects due to ACTC1 mutation4; b) the variant strongly cosegregated with the phenotype, and c) the molecular consequences of the variant were considered probably pathogenic. Further functional information obtained from animal models may be valuable to confirm the causal role of the ACTC1I289T mutation.
In summary, we offer the phenotypical description of a family with LVNC caused by the highly penetrant, novel, heterozygous ACTC1I289T mutation. Remarkably, in the literature this is the third ACTC1 mutation causing LVNC, and associated ostium secundum atrioseptal defect in some affected family members.
FUNDINGThis work was supported by the Instituto de Salud Carlos III (PI11/00019, CP09/00065 and RD12/0042/0029), the Generalitat Valenciana (PROMETEO 2011/027), and the Agence Nationale de la Recherche (ANR-13-BSV1-0023-03).
We thank the patients for taking part in the study and Biobanco La Fe for its technical support (PT13/0010/0026).