Keywords
INTRODUCTION
Aortic coarctation is a common congenital defect that accounts for 5% to 8% of cardiovascular malformations.1 Surgery is a good therapeutic option for these patients. However, regardless of the technique used, a significant percentage of patients develop long-term complications,2,3 which range from recoarctation to aneurysms, dissection, and aortic rupture.4,5
Such complications are not rare, and the development of aneurysms has been described in 7% to 38% of patients, with a high mortality associated with rupture.2,6 Recoarctation after an initial successful operation is also not rare and has an incidence of 3% to 26%.2,4,5
Treatment of complications after the first operation is problematic, and repeat surgery has been associated with mortality rates ranging from 14% to 23%.2,4 Although the endovascular approach is increasingly used to treat patients with congenital coarctation,7 its usefulness after corrective surgery is not well established, particularly for treatment of the aneurysms these patients develop, which often affect the aortic wall. The usefulness of intravascular ultrasound to characterize the anatomic defect and to guide treatment in these procedures is also not well defined.
We describe our experience with endovascular treatment in 4 patients who presented complications (1 recoarctation and 3 aneurysms) following corrective surgery of aortic coarctation.
PATIENT 1
A 52-year-old woman who had undergone aortic coarctation repair consisting of interposition of a Dacron graft at age 17 was referred for hypertension (HT) refractory to treatment. Magnetic resonance angiography revealed stenosis that produced a lumen diameter of 3 mm at the graft anastomosis with the distal aorta. Catheterization was performed and showed a 100 mmHg gradient at the stenosis.
To determine the cause of the stenosis, rule out negative remodeling in the distal anastomosis, and guide the corrective procedure, intravascular ultrasound (IVUS) was performed using a nonorientable 9-MHz, 9-French Ultra ICE catheter (Boston Scientific, California, United States) connected to a Galaxy console (Boston Scientific).
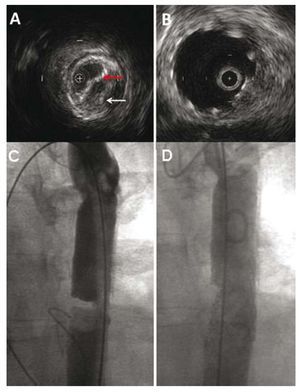
Extrinsic compression of the graft produced a lumen diameter of 4´11 mm; however, the outer diameter of the aorta remained unchanged (15 mm) (Figure 1). The outer diameter of the segment of healthy distal aorta was equal to that observed in the area of greatest stenosis (15 mm).
Figure 1. Case 1. A: intravascular echocardiogram during the procedure; note how recoarctation (white arrow) has deformed the stent wall (red arrow). B: final outcome in the same region. C: pretreatment angiography. D: posttreatment angiography.
A Genesis XD stent (Cordis Endovascular, Miami, Florida, United States) of 10´27 mm mounted on a BIB balloon of 14´35 mm was then implanted. After the procedure, the gradient was 3 mmHg and IVUS showed a stent that rested properly against the aortic wall (Figure 1B).
The patient presented no complications on follow-up, and magnetic resonance imaging (MRI) 6 months after the procedure showed a well-positioned, expanded stent with no associated aneurysm.
PATIENT 2
A 55-year-old woman had undergone preductal aortic coarctation repair at age 18 consisting of interposition of a triangular Dacron patch. In the immediate postoperative period, she remained normotensive. Thirty-seven years later, she was referred for refractory HT. At the time of consultation, she was asymptomatic and the physical examination was normal.
A computed tomography (CT) scan showed a 5´35-mm pseudoaneurysm in the inner part of the aortic arch that extended toward and occupied the aortopulmonary window and rested on the pulmonary trunk and the left pulmonary artery. IVUS revealed a large mouth of 4.7 mm facing the origin of the left subclavian artery.
A decision was made to implant a graft with exclusion of the left subclavian artery, and the patient tolerated a 30-minute test occlusion on the left vertebral artery.
A 95´26-mm aortic graft (Relay Thoracic Stent Graft, Bolton Medical, Barcelona, Spain) was implanted by dissection of the right femoral artery with the patient under general anesthesia. The covered proximal portion was placed behind the origin of the left carotid artery and the distal portion in the descending thoracic aorta.
At the last follow-up (8 months after the procedure), the patient was asymptomatic and the CT scan confirmed sealing of the aortic aneurysm.
PATIENT 3
A 35-year-old man was operated at age 18 for postductal aortic coarctation by placement of a rhomboidal Dacron patch. Following surgery, he presented persistent HT and was placed long-term nevibolol therapy. He was referred for the development of uncontrollable HT.
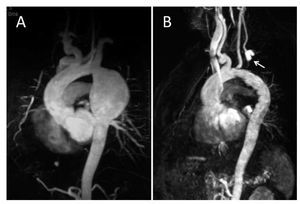
MRI showed considerable dilatation of the thoracic aorta of 62´55´80 mm that began 4 mm from the origin of the left subclavian artery (Figure 2A).
Figure 2. Case 3. Magnetic resonance. A: large aneurysm after the origin of the left subclavian artery. B: after the procedure; the origin of the left subclavian artery is occluded by the graft and the vessel is filled through the left carotid artery (arrow).
A 24´150-mm percutaneous endovascular graft (Relay Thoracic Stent Graft) was implanted through a right femoral approach and covered the origin of the left subclavian artery. After the procedure, the patient presented good blood pressure control. At the last follow-up visit (3 years after the procedure), he remained asymptomatic and MRI showed a well-positioned stent with no blood flow to the interior of the aneurysm (Figure 2B).
PATIENT 4
A 37-year-old man had undergone surgery at age 13 for preductal aortic coarctation with placement of a rhomboidal Dacron patch. At age 30 he was reoperated for recurrent coarctation with a 65-mmHg gradient and development of an aneurysm. At a follow-up visit, the patient was found to have an aneurysmal dilatation with a length of 5 cm and height of 3.5 cm that affected the aortic arch and involved the origin of the left subclavian artery immediately after the origin of the left carotid artery.
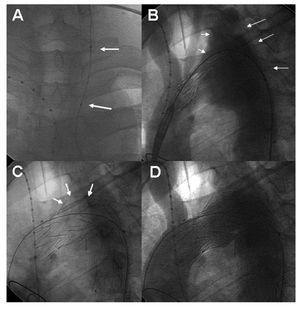
A decision was made to implant an endovascular graft after confirming the integrity of the circle of Willis. Before the procedure, good tolerance was confirmed after a 15-minute test occlusion of the left carotid artery, followed by drug-induced hypotension (Figure 3A).
Figure 3. Case 4. A: balloon test occlusion (arrows) of the left carotid artery. B: angiography before stent placement; aneurysmal dilatation of the aortic arch, involving the origin of the left subclavian artery (arrows). C: following implantation, note that the graft wall is bulging at the level of the aneurysm (arrows). D: angiography after the procedure; no contrast leakage or contrast flow to the left carotid and subclavian arteries (in comparison with B) is seen.
A 34´145-mm stent (Relay Stent Graft) was implanted using a percutaneous approach via the right femoral artery with occlusion of the origin of the left carotid and subclavian arteries (Figure 3). After the procedure, the patient did not require antihypertensive treatment. A CT scan carried out 3 years later showed good aortic stent positioning and no aneurysm filling.
DISCUSSION
Surgical repair of aortic coarctation includes resection of the stenotic segment and aortic repair by direct anastomosis or aortoplasty using a subclavian artery or synthetic patch.
It has been shown that there are fewer long-term complications with the direct anastomosis technique than with other types of repair. However, long-term complications are not uncommon, irrespective of the surgical technique used.2-6
Conservative treatment of the complications, particularly aneurysms, is unpredictable. Knyshov et al6 reported an aneurysm rupture rate of 100% at 15 years, whereas Cohen et al2 described 7% of deaths from aortic complications following coarctation surgery.
The treatment of these patients is complex and challenging because repeat surgery has been associated with high mortality and significant morbidity that includes paraplegia, bleeding, and paralysis of the recurrent nerve.6
The endovascular approach yields good outcomes in atherosclerotic thoracic aneurysm repair and appears to be a good alternative for surgical complications after coarctation. Several groups have reported small series with excellent results.8-10
As in other series, none of our patients died or experienced severe complications related to the procedure.
In our experience, 3 aspects should be stressed: a) occlusion of supra-aortic trunks; b) problems derived from the vascular access; and c) the use of intra-aortic IVUS.
Occlusion of the origin of the left subclavian artery was necessary in 3 of our patients, 1 of whom also required occlusion of the left carotid artery. Because the left vertebral artery and the circle of Willis were patent in all patients, the procedure did not need to include a vascular bypass.
It has been shown that intentional occlusion of the origin of the left subclavian artery is a safe process, provided that competent collateral perfusion is first documented.7,11 Even when the other vessels are competent, neurologic symptoms or claudication of the left arm can develop.7 In order to avoid these complications, 2 of our patients underwent test occlusion (vertebral artery in 1; subclavian in 1). In all cases, close follow-up is needed to detect long-term abnormalities.
Vascular access may be difficult in these patients, as a history of coarctation means that ileofemoral arteries are not fully developed in many cases. A complication-free percutaneous approach was possible in 3 of our patients. The other patient presented a 7-mm femoral artery documented by IVUS and, therefore, dissection of the vessel was preferred. This aspect highlights the need for a multidisciplinary evaluation to tailor the approach for each individual patient.
Lastly, we used intra-aortic IVUS for a more complete assessment of 2 of these complex cases. In our experience, the technique made it possible to decide on treatment by determining the true aorta size and the nature of the problem and to evaluate the results during the procedure.
In summary, in our experience, endovascular treatment of postoperative complications of aortic coarctation appears to be safe and highly successful. However, extensive clinical and imaging follow-up is needed to determine the usefulness of this approach and to detect potential long-term complications.
Correspondence: Dr. P. García-Pavía.
Servicio de Cardiología. Hospital Universitario Puerta de Hierro. Manuel de Falla, 1. 28222 Majadahonda. Madrid. España.
E-mail: pablogpavia@yahoo.es
Received December 22, 2008.
Accepted for publication June 18, 2009.