Keywords
INTRODUCTION
The heart is the main organ affected by Chagas disease. Some 20%-30% of patients suffer chronic chagasic cardiomyopathy (CCC), characterized by heart failure, arrhythmias and sudden death. Microvascular lesions have been implicated in the pathogeny of CCC; these cause multiple ischemic foci,1 leading to an increase in the concentration of adenosine, the cardiovascular effects of which are mediated by G protein-coupled receptors.2
The therapeutic properties of adenosine have been associated with proarrhythmic effects. In patients with structurally normal hearts, adenosine can induce atrial fibrillation, an increase in atrioventricular (AV) node conduction, and nonsustained polymorphic ventricular tachycardia.3 In children and adults with prolonged QT syndrome, it can induce torsade de pointes (TP)-type ventricular tachycardia.4
Sudden death in Chagas disease is owed to ventricular fibrillation (VF) conditioned by humoral, vascular and structural anomalies. Ventricular fibrillation has been related to ventricular repolarization abnormalities reflected in a long QT interval5 and early- (the R- on- T phenomenon) and late-coupled premature ventricular complexes.6
Focal myocardial ischemia in Chagas disease induces the release of adenosine, which unleashes fatal arrhythmias facilitated by repolarization abnormalities. The present work investigates the role of the adenosinergic system in inducing of ventricular arrhythmia.
METHODS
Sample
Twenty-eight Sprague-Dawley rats were divided into 2 groups: a control group of healthy rats (n=14) and a group with CCC (n=14; confirmed histopatholologically to have chronic myocarditis) (Figure 1).
Figure 1. Histopathological and electrocardiographic evidence of chronic chagasic cardiomyopathy. The sampled heart sections were fixed in PBS-formalin, set in paraffin blocks and stained with hematoxylin and eosin. A shows fibrosis; B shows the mononuclear inflammatory infiltrate. The examples shown are for healthy (C) and chagasic rats (D-E) anesthetized with pentobarbital (40 mg/kg) before the extraction of the heart. Notice that the chagasic rats show nodal extrasystole (D) and atrioventricular block (E).
Isolated Heart
A Langendorff continuous retrograde perfusion system was set up, involving a modified Krebs-Henseleit-type solution containing 10 nmol of isoproterenol, pH 7.35-7.45, bubbled with 95% O2 and 5% CO2 at 37°C. The isoproterenol was used to maintain high adrenegic tone and to facilitate the appearance of arrhythmias. Electrocardiographic readings were taken in bipolar mode; the electrodes were located at the tip of the right atrium. The effect of adenosine was tested at increasing concentrations (10-3333 µmol). The effects of selective A1 (1,3-dipropyl-8-cyclopentylxanthine [DPCPX]), A2a (8-chlorostyril caffeine [8-CSC]) and A2b (alloxazine [ALLO]) receptor antagonists, and the A3 antagonist VUF 5574, were assayed in the presence and absence of 0.33-1 mmol adenosine.
Data Analysis
Data were analyzed using the Student t test or ANOVA for paired samples as required, followed by the Bonferroni test. Qualitative variables were analyzed using the χ2 test. Significance was set at P<.05.
RESULTS
Electrocardiograms were recorded with pentobarbital anesthesia before setting up the Langendorff preparation; these showed chagasic heart rhythm abnormalities (nodal extrasystoles) in five rats, as well as conduction abnormalities (AV and fascicular block). Three control rats showed RR variability only (Figure 1).
Electrocardiographic Variables
Tables 1 and 2 show the PR, QRS and QT intervals obtained with increasing doses of adenosine and with DPCPX in the presence of 333 µmol of adenosine. The results show that adenosine induced an increase in the QT interval that was reverted with DPCPX. No significant differences were seen for the PR and QRS intervals.
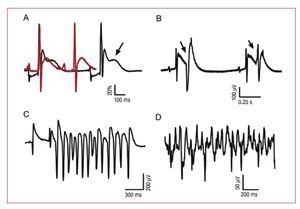
Figure 2A shows the increase in the QT interval and the presence of early depolarizations (arrow). Figure 2B shows premature ventricular complexes emerging from the T wave. Figure 2C shows the TP phenomenon emerging from a prolonged T wave.
Figure 2. Prolongation of the QT interval induced by early post-depolarizations, R on T phenomena, torsade de pointes and ventricular fibrillation. A: recordings obtained for chagasic hearts in the presence (black line) and absence (red line) of 100 µmol adenosine; note the prolongation of the QT interval and early depolarizations (arrow); the amplitude was normalized to facilitate the superimposition of the recordings. B: premature ventricular complexes arising out of the T wave (R on T phenomenon). C: torsade de pointes. D: ventricular fibrillation.
Rhythm Abnormalities
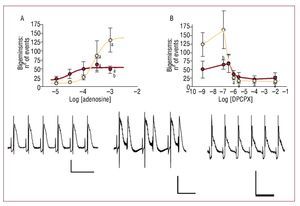
Adenosine induced a significant increase in the number of nodal and ventricular extrasystoles and bigeminisms in both groups in a dose-dependent manner; these reverted with DPCPX. The members of the control group suffered more bigeminisms (P<.05) (Figure 3).
Figure 3. Bigeminisms induced by A1 receptors. Beige represents the control, red the treatment. All recordings were obtained from the same chagasic rat. A: adenosine dose-response curve. B: dose response curve for DPCPX in the presence of 333 µmol adenosine; recordings are baseline (left), perfusion with adenosine (middle) and perfusion with DPCPX-adenosine (right); the vertical line represents 100 µV and the horizontal 0.5 s. aP<.05 with respect to baseline.bP<.05 between groups at the dose indicated.
Ventricular Fibrillation
Adenosine induced VF in 50% (n=7) of chagasic hearts and 7.14% (n=1) of healthy hearts (Figure 2D). The chagasic hearts were associated with the possibility of fibrillation (P<.05). No VF was seen in the presence of DPCPX and 8-CSC, but 8-CSC induced ventricular tachycardia in 50% (n=2) of the hearts. Neither alloxazine 1 mmol nor VUF 176 nmol prevented VF.
DISCUSSION
Congenital4 and acquired7 prolonged QT syndrome is a predisposing factor to serious, adenosine-induced ventricular tachycardia. In such patients the bradycardia induced by adenosine causes TP, which can degenerate into ventricular fibrillation.8 Certainly, TP is an uncommon type of arrhythmia, and its appearance depends on the existence of bradycardia9 and increased adrenergic discharge.10
The intracellular overload of Ca2+ induced by isoproterenol11 and adenosine, mediated by the A2a receptors2 activates the Na+/Ca2+exchange mechanism, which in turn prolongs the QT interval. Such conditions would favor the appearance of early and late depolarizations. The former are exacerbated by low heart rates and contribute to the appearance of polymorphic ventricular arrhythmias associated with a long QT interval.11 It is known that the simultaneous administration of adenosine and isoproterenol can induce arrhythmias in patients prone to atrial fibrillation.12
In the present work, the administration of DPCPX led to a significant reduction in the length of the QT interval and the reversion of arrhythmia. The relationship between the A1 receptors and the induction of arrhythmias has been demonstrated in transgenic models.13
Adenosine was able to induce VF in the chagasic rats, an effect avoided with the administration of DPCPX and 8-CSC, indicating the mediation of the A1 and A2a receptors. It is known that the probability of VF appearing, facilitated by hypoxia and reoxygenation phenomena, is reduced with DPCPX,14 confirming that the A1 receptors are involved in VF. It is also known that the ability of adenosine to induce VF is blocked by the depletion of catecholamines in the heart,15 confirming that adenosine induces VF under hypoxia-reoxygenation conditions via a mechanism facilitated by the activation of the adrenergic system.
In CCC, abnormalities of the coronary microcirculation lead to cycles of hypoxia, ischemia and reoxygenation.1 This would condition the chagasic heart to the profibrillatory action of adenosine, which, in the present model, was favored by the sustained activation of the beta-adrenergic receptors. The medical importance of this work lies in its linking of the arrhythmias associated with the prolonged QT interval with the A1 adenosinergic system. This opens up the possibility of studies designed to investigate the therapeutic effect of A1 antagonists in these arrhythmias.
This work was funded by CDCHT, UCLA, Barquisimeto-Venezuela, Project Nº 001-ME-2005.
Correspondence: Dr. R. Bonfante-Cabarcas.
Unidad de Bioquímica. Decanato de Ciencias de la Salud. Universidad Centro-Occidental Lisandro Alvarado.
Avda. Libertador con Andrés Bello. 3001 Barquisimeto. Venezuela.
E-mail: rcabarca@ucla.edu.ve
Received April 27, 2009.
Accepted for publication June 18, 2009.