We present the case of a 62-year-old woman, an ex-smoker with high blood pressure and hyperthyroidism, who had been diagnosed in 1992 with tricuspid endocarditis and the Gerbode defect, a left ventricle to right atrium shunt.1 The patient reported mild dyspnea on exertion, and spirometry showed moderate obstruction.
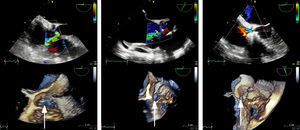
After 23 years, the patient developed symptoms of predominantly right-sided heart failure. Echocardiography and catheterization after maximum tolerated medical treatment showed dilation of the right chambers and left ventricle, moderate biventricular dysfunction (left ventricular ejection fraction = 40%), the Gerbode defect (peak velocity = 5 m/s and Qp/Qs = 2.1), and significant pulmonary hypertension (50/20/30 mmHg) with no transpulmonary gradient. The defect measured 20 × 6 mm on 3-dimensional echocardiography (Figure 1 and video 1A in Appendix B of the supplementary material) and 5.5 mm on angiography (Figure 2 and video 1B in Appendix B of the supplementary material).
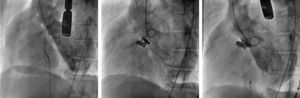
Ventricular angiography showing the Gerbode defect before treatment (left), the residual defect after the first procedure with 2 Nit-Occlud Lê VSD coils measuring 12 × 8 and 10 × 6 mm (center), and the minimal defect after the second procedure with a 6 mm Amplatzer VSD device and a 4 × 4 mm ADO II device (right).
Although catheter-based closure is now the preferred treatment at many centers, surgical repair remains the norm and treatment options for this patient were first discussed in a medical-surgical session. Surgery was rejected due to the elevated comorbidity and biventricular dysfunction, and the decision was therefore taken to close the defect percutaneously with the Nit-Occlud Lê VSD coil duct occluder.2 After an arteriovenous circuit was established, a 12 × 8 mm device was implanted through a 7-Fr delivery sheath (video 2A in Appendix B of the supplementary material). A notable residual defect persisted after this procedure, and a second device was therefore implanted, measuring 10 × 6 mm. Release of the 2 devices markedly reduced the short circuiting (residual defect < 5 mm) and triggered a 20 mmHg drop in pulmonary pressure (Figure 1, Figure 2, and video 2B in Appendix B of the supplementary material), confirming a major contribution from pulmonary hyperflow.
The patient later developed intense choluria, with high serum levels of bilirubin and lactate dehydrogenase (LDH). Hemolysis was confirmed by undetectable serum haptoglobin, low hemopexin, and a negative Coombs test. The patient had an indolent clinical course with recurrent hemolysis requiring repeated transfusions every 48 to 72hours and hemodialysis to treat acute kidney failure. After 1 month, the hemolytic episodes persisted, and after the surgical option was again rejected, percutaneous intervention was undertaken in a further attempt to close the small residual defect.
A 6-Fr sheath was inserted through the defect to implant a 6-mm Amplatzer VSD device (video 3 A and B in Appendix B of the supplementary material). A slight residual defect persisted, and a 0.014-inch guidewire was therefore used to position a 4-Fr sheath, through which a 4 × 4 mm Amplatzer Duct Occluder II Additional Sizes device was implanted. This resulted in a minimal residual defect, and the 2 devices were released (Figure 1, Figure 2, and videos 3B-D in Appendix B of the supplementary material). After 6 months, the patient remained asymptomatic, with no hemolysis and restored kidney function.
The Gerbode shunt was first described as a congenital defect,1 but this type of defect frequently occurs as a complication of surgical or percutaneous cardiac procedures3 and after tricuspid valve endocarditis.3,4 Hemodynamic repercussions are an indication for defect closure, often performed via the percutaneous route. Gerbode defects are located close to the His bundle or the aortic valve, and the occluder device must therefore be selected with care to avoid interference. For our patient, we chose Nit-Occlud Lê VSD coil duct occluders because of their efficacy and safety in the closure of perimembranous defects.2 The high flexibility of these devices reduces the risk of tearing delicate structures; moreover, these devices adapt to complex and irregular defect morphologies and reduce the risk of interference with valve function. To our knowledge, this is the first case report of the use of this device to treat a Gerbode defect and is also the first to report simultaneous implantation of 2 devices of this type.
Hemolysis is a recognized complication of surgical and percutaneous procedures for closing heart defects. The risk of hemolysis is increased by incomplete closure, and although it can be transient,2,5 hemolysis often persists, a situation that requires reintervention.6 The high prevalence of hemolysis associated with small residual septal defects is explained by the large pressure difference between the heart chambers. It is also possible that the structure of the devices used might contribute to this complication; however, this hypothesis is not supported by the published data, as the incidence of hemolysis requiring reintervention is < 5%.2
Due to concerns about possible interference with aortic valve motion, we selected a smaller device than indicated by initial modeling. This may have contributed to the persistence of the defect; however, we cannot distinguish the potential influence of device size from effects due to defect morphology, tissue tearing, or the device design itself. For the second procedure, concerns about device design prompted us to select an Amplatzer VSD device because of the small size of its closing disks and an Amplatzer Duct Occluder II Additional Sizes device because of its minimal profile.
It is often wise to apply Voltaire's aphorism “perfect is the enemy of good enough”; however, for the closure of Gerbode defects, we must insist on excellence, in the form of the most complete closure possible, in order to avoid complications.
CONFLICTS OF INTERESTJ.L. Zunzunegui Martínez is a proctor for PFM Medical and St. Jude Medical.