The hallmark clinical feature of heart failure (HF) is a severe reduction in exercise capacity, which limits patients’ activities of daily living and is a crucial determinant of increased risk of adverse outcomes.1 In patients with chronic HF and reduced ejection fraction (HFrEF), sacubitril/valsartan reduced the risk of the composite of cardiovascular death or first hospitalization for HF by 20% compared with enalapril at a median follow-up of 27 months.2 However, evidence supporting the role of this treatment combination for improving short-term functional capacity is lacking.
In this work, the primary endpoint was to evaluate the short-term effects of sacubitril/valsartan on maximal exercise capacity evaluated by peak oxygen consumption (peak VO2) in stable patients with symptomatic HFrEF. The secondary endpoint included changes in ventilatory efficiency during exercise (VE/VCO2 slope).
From March 1, 2017 to July 1, 2017, we prospectively studied a cohort of patients with chronic HF, visited in the HF unit of a tertiary center in Spain. The inclusion criteria were: a) left ventricular ejection fraction < 40%; b) stable New York Heart Association functional class ≥ II; c) ability to perform a valid baseline exercise test, and d) prior treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
For eligible patients, treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker was replaced by sacubitril/valsartan. All patients provided informed consent and the protocol was approved by the research ethics committee in accordance with the principles of the Declaration of Helsinki and national regulations.
At each visit (baseline assessment and after 30-day initiation of sacubitril/valsartan), we registered demographic information, medical history, vital signs, 12-lead electrocardiogram, cardiopulmonary exercise testing), quality of life (Minnesota Heart Failure Living questionnaire), standard laboratory data, and pharmacological treatments. Doses of sacubitril/valsartan were prescribed according to established recommendations.1 By protocol, no treatment changes occurred between the 2 visits.
Maximal functional capacity was evaluated with incremental and symptom-limited cardiopulmonary exercise testing (CORTEX Metamax 3B) on a bicycle ergometer, beginning with a workload of 10W and increasing stepwise at 10-W increments every 1minute. Gas exchange data and cardiopulmonary variables were averaged every 10-second values. Peak VO2 was considered the highest value of VO2 during the last 20seconds of exercise. The VE/VCO2 slope was determined by measuring the slope across the entire course of exercise.
Continuous variables are expressed as mean ± standard deviation or median [interquartile range] as appropriate; discrete variables as percentages. In an ANCOVA design, changes in peak VO2 were tested with a mixed-effects model for repeated-measures. The model included as covariates the sacubitril/valsartan doses and the baseline value of peak VO2. A 2-sided P value of < .05 was set as the criterion for statistical significance.
A total of 33 consecutive HFrEF patients were screened for eligibility and 16 were finally included in this study (Figure 1 of the supplementary material). The main reasons for ineligibility were baseline systolic blood pressure < 100mmHg (n = 7), estimated glomerular filtration rate < 30mL/min/1.73 m2 (n = 5), and orthopedic/neurological inability to perform a valid cardiopulmonary exercise test (n = 4).
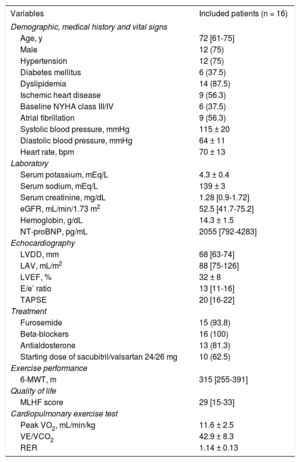
Median [interquartile range] age was 72 years [61-75], 75% were men, 56.3% had prior ischemic heart disease, and 37.5% were in New York Heart Association functional class III. The mean ± standard deviation of left ventricular ejection fraction, peak VO2, and VE/VCO2 slope were 32 ± 8%, 11.6 ± 2.5mL/min/kg, and 42.9 ± 8.3, respectively. The starting dose of sacubitril/valsartan was 24/26mg in 10 patients (62.5%). Baseline characteristics are presented in the Table. Using raw values, an improvement of at least 10% of the baseline peak VO2 and VE/VCO2 slope were found in 5 (31.3%) and 4 (25%) patients, respectively.
Baseline Characteristics of the Study Population
| Variables | Included patients (n = 16) |
|---|---|
| Demographic, medical history and vital signs | |
| Age, y | 72 [61-75] |
| Male | 12 (75) |
| Hypertension | 12 (75) |
| Diabetes mellitus | 6 (37.5) |
| Dyslipidemia | 14 (87.5) |
| Ischemic heart disease | 9 (56.3) |
| Baseline NYHA class III/IV | 6 (37.5) |
| Atrial fibrillation | 9 (56.3) |
| Systolic blood pressure, mmHg | 115 ± 20 |
| Diastolic blood pressure, mmHg | 64 ± 11 |
| Heart rate, bpm | 70 ± 13 |
| Laboratory | |
| Serum potassium, mEq/L | 4.3 ± 0.4 |
| Serum sodium, mEq/L | 139 ± 3 |
| Serum creatinine, mg/dL | 1.28 [0.9-1.72] |
| eGFR, mL/min/1.73 m2 | 52.5 [41.7-75.2] |
| Hemoglobin, g/dL | 14.3 ± 1.5 |
| NT-proBNP, pg/mL | 2055 [792-4283] |
| Echocardiography | |
| LVDD, mm | 68 [63-74] |
| LAV, mL/m2 | 88 [75-126] |
| LVEF, % | 32 ± 8 |
| E/e’ ratio | 13 [11-16] |
| TAPSE | 20 [16-22] |
| Treatment | |
| Furosemide | 15 (93.8) |
| Beta-blockers | 16 (100) |
| Antialdosterone | 13 (81.3) |
| Starting dose of sacubitril/valsartan 24/26 mg | 10 (62.5) |
| Exercise performance | |
| 6-MWT, m | 315 [255-391] |
| Quality of life | |
| MLHF score | 29 [15-33] |
| Cardiopulmonary exercise test | |
| Peak VO2, mL/min/kg | 11.6 ± 2.5 |
| VE/VCO2 | 42.9 ± 8.3 |
| RER | 1.14 ± 0.13 |
bpm, beats per minute; eGFR, estimated glomerular filtration rate using the Modification of Diet in Renal Disease formula; LAV, left atrial volume by biplane modified Simpson; LVDD, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; MLHF, Minnesota Living with Heart Failure Questionnarie score; 6-MWT, 6-minute walk test; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association functional class; peak VO2, peak oxygen consumption; RER, respiratory exchange ratio; TAPSE, tricuspid annular plane systolic excursion; VE/VCO2 slope, relationship between minute ventilation and the rate of CO2 elimination.
Data are expressed as No. (%), mean ± standard deviation or median [interquartile range].
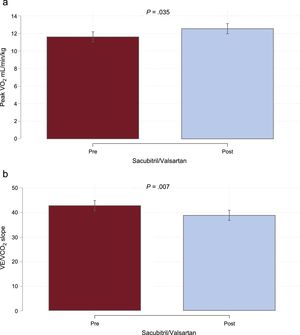
Compared with baseline, peak VO2 increased significantly at 30 days (+Δ = 0.92; 95% confidence interval, 0.06-1.77; P = .035), which corresponded to a 7.9% increase from the baseline value (Figure A). Likewise, a significant improvement in VE/VCO2 slope was also found at 30 days (-Δ = 3.89; 95% confidence interval, 6.70-1.07; P = .007), which corresponded to a 9.1% of reduction, as shown in Figure B. In parallel, a significant improvement in other surrogates of severity such as quality of life and N-terminal pro-B-type natriuretic peptide (32.22% and 5.29% of reduction, respectively) was registered (Figure 2 of the supplementary material). No significant changes were detected in estimated glomerular filtration rate (Figure 2 of the supplementary material).
Thirty-day effects of sacubitril/valsartan on CPET parameters. A: changes in peak VO2. B: changes in VE/VCO2 slope. CPET, cardiopulmonary exercise test; peak VO2, peak oxygen consumption; VE/VCO2 slope, minute ventilation/carbon dioxide production. Adjusted for baseline values of both exposures.
To the best of our knowledge, this is the first study suggesting that the initiation of sacubitril/valsartan, mostly at low doses, could lead to a short-term increase in peak VO2. Interestingly, this beneficial effect was associated with a significant improvement in other cardiopulmonary exercise testing surrogates of severity such as ventilatory efficiency. Although the mechanisms by which sacubitril/valsartan might improve early exercise capacity in HFrEF remain unclear, we speculate that neprilysin inhibition mediated by sacubitril would acutely amplify the hemodynamic effects of natriuretic peptides and other vasoactive peptides,3 resulting in an improvement of short-term exercise tolerance.
The main limitation of this study is the small sample size and the lack of a control group. However, we believe these encouraging findings open a new research path aimed at exploring the pathophysiological mechanism by which sacubitril/valsartan improves exercise tolerance in HFrEF. Indeed, a clinical trial evaluating the effect of sacubitril/valsartan on 6-month Exercise Tolerance in Patients With Heart Failure (NEPRIExTol) is currently ongoing (NCT03190304).
FUNDINGThis work was supported in part by grants from CIBER CV 16/11/00420, 16/11/00403; FEDER and PIE15/00013.