The therapeutic options for patients with advanced heart failure have changed significantly in recent years, due not only to pharmacological advances, but also to the advent of durable left ventricular assist devices (LVAD).1
As LVAD therapy became established in heart transplant centers,2 it also began to be implemented at tertiary referral hospitals lacking a transplant unit. This article presents the initial results from Salamanca University Hospital and Álvaro Cunqueiro Hospital, 2 Spanish nontransplant centers that have together acquired some experience with this approach.
To date, these hospitals have implanted 11 LVADs, 8 at Salamanca University Hospital and 3 at Álvaro Cunqueiro Hospital. Of the implanted devices, 9 were HeartWare and 2 were HeartMate III. LVAD implantation was the destination therapy in 10 patients (90.9%) and in the other patient was used as a bridge to transplant (table 1). In December 2014, the unit at Salamanca University Hospital was the first in Spain to implant an LVAD in a woman,3 and after 4.5 years this patient now has the longest LVAD support time in this country. The median age of the patients at the time of implantation was 68 years [interquartile range, 62-74 years], and the most frequent heart condition was ischemia (8 patients [72.7%]).
Demographic characteristics, implantation date, LVAD indication, underlying heart disease, complications, and progression of patients receiving a long-duration left ventricular assist device
| Age, y | Sex | Implantation date | Treatment type | Underlying heart disease | Thrombotic events | Major bleeding events | RV failure | Driveline infection | Death |
|---|---|---|---|---|---|---|---|---|---|
| 73 | W | 2014, 12 | Destination | Ischemic | No | No | No | Superficial (chronic) | No |
| 66 | M | 2015, 11 | Destination | Ischemic | Ischemic stroke | Rectal bleeding | No | Superficial (resolved) | No |
| 68 | W | 2016, 05 | Destination | Ischemic | No | No | Acute | No | Yes (7 d) |
| 65 | W | 2016, 11 | Destination | Ischemic | No | No | Acute | No | Yes (after HT) |
| 64 | M | 2017, 05 | Destination | Dilated cardiomyopathy | No | Hematuria | Chronic | Superficial (under treatment) | No |
| 73 | M | 2017, 12 | Destination | Mixed (ischemic and valvular) | Pump thrombosis | No | No | Superficial (resolved) | No |
| 65 | M | 2018, 02 | Bridge to transplant due to pulmonary hypertension | Ischemic | Ischemic stroke (right MCA) | No | No | Superficial (under treatment) | No |
| 69 | M | 2018, 02 | Destination | Dilated cardiomyopathy | Ischemic stroke (LVAD thrombosis) | Subarachnoid hemorrhage | No | No | Yes (> 7 d) |
| 73 | M | 2018, 04 | Destination | Ischemic | No | No | Chronic | No | No |
| 62 | M | 2018, 11 | Destination | Valvular | No | No | No | No | No |
| 74 | M | 2018, 12 | Destination | Ischemic | No | No | No | No | No |
LVAD, left ventricular assist device; HT, heart transplant; M, man; MCA, middle cerebral artery; RV, right ventricle; W, woman.
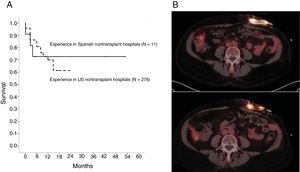
Of the 11 patients, 3 (27.3%) died after LVAD implantation. Of the deaths, 2 were due to right ventricular (RV) failure: one in the first week after LVAD implantation and the other following heart transplant required after several months of poor progress. The third death was due to a subarachnoid hemorrhage arising as a complication of thrombolysis after pump thrombosis and ischemic stroke. Cumulative survival in our cohort is shown in figure 1A, together with survival data for a cohort of destination-therapy LVAD patients undergoing the procedure at nontransplant hospitals in the United States (N=276).4 The comparison shows that the data from the 2 cohorts are superimposable.
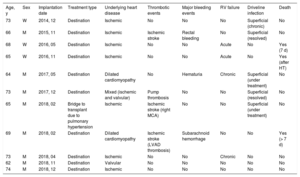
LVAD implantation can increase survival and improve patient quality of life; however, the procedure is associated with multiple complications whose prevalence increases with device support time. The most frequent infectious complication is infection of the driveline. In the patient with the longest LVAD support time, superficial driveline infection was detected within a few months after device placement. Analysis by positron-emission tomography–computed tomography (PET-CT) confirmed proximal extension of the infection despite several rounds of treatment with broad-spectrum antibiotics (representative images are shown in figure 1B). The patient was therefore placed on dalbavancin and subsequently showed excellent progress. At the time of writing, she has a superficial driveline infection managed with oral antibiotics. Of the other patients, 4 (36.4%) have superficial infections managed with oral antibiotics and topical treatments.
One of the most dreaded complications of LVAD implantation is pump thrombosis. In our cohort, partial pump thrombosis has affected 2 patients (18.2%). The first was the patient described above who died after a subarachnoid hemorrhage. In the second patient, pump thrombosis was associated with closure of a mechanical aortic valve prosthesis 15 months after LVAD placement and was first evident from hematuria and a significant increase in power consumption. Anticoagulation with argatroban reduced hemolysis markers and power consumption; however, the patient's condition worsened at 96hours, and he was therefore treated by systemic thrombolysis with recombinant tissue plasminogen activator. His condition subsequently improved, with normalization of both parameters and without bleeding complications.
Within the cohort, 3 patients (27.3%) had an ischemic stroke not treated by thrombectomy. The first was the patient with pump thrombosis who died. Another patient had a right middle cerebral artery (MCA) stroke within the first 30 days of LVAD support, likely related to his high blood pressure readings; the patient recovered without sequelae. The third patient had a left MCA stroke after 2.5 years of LVAD support; he recovered from the stroke with minimal residual right-sided hemiparesis that does not interfere with his ability to live independently. The same patient has had 2 episodes of lower gastrointestinal bleeding, but endoscopy has shown no evidence of bleeding lesions, and since these events he has made good progress. Another patient had hematuria secondary to radiation cystitis, requiring hemotherapy and adjustments to the antithrombotic therapy.
In addition to the 2 patients with acute RV failure who died, 2 patients (18.2%) have chronic RV dysfunction; both patients have had isolated incidents of systemic congestion and responded well to diuretic therapy, with 1 of the patients requiring occasional levosimendan infusions. None of the patients has shown evidence of severe aortic regurgitation or notable systemic failure.
Patients with an LVAD can require surgery during follow-up. In our cohort, 2 patients (18.2%) needed cardiac interventions: one patient who had previously undergone the Ross procedure required aortic valve replacement, and the other required tricuspid annuloplasty. Another 2 patients underwent inguinal herniorrhaphy with intravenous heparin bridge therapy, with no complications.
The data presented here are comparable to findings in American centers4 and support the safety of LVAD implantation in nontransplant hospitals when performed by a multidisciplinary team of experienced cardiologists and cardiac surgeons. The cardiologists should have completed their training at high-volume international centers (at least 3 months in our experience), and cardiac surgeons should be supported by a proctor during device implantation; moreover, the team should work in close coordination with the referral transplant hospital. In the future, the creation of a national registry of LVAD implantation activity at nontransplant centers such as ours is certain to help practitioners to share experiences and improve results.