Hydroxychloroquine is widely used to treat rheumatoid arthritis and lupus erythematosus. When administered orally, the drug has high bioavailability, long elimination half-life (30-60 days), and large volume of distribution and steady-state concentrations are reached after 4 to 6 months of treatment.1 Retinal toxicity is a well-known adverse effect and screening recommendations have been established for its prevention.2 Rarely, the drug can cause cardiomyopathy, which can be fatal unless it is suspected at an early stage. Hydroxychloroquine induces phospholipidosis (phospholipid accumulation in the cytoplasm) through inhibition of lysosomal phospholipase to form indigestible complexes visible under an electron microscope. Depending on their morphology, these are denoted myeloid bodies (forming concentric layers) or curvilinear bodes (comma shape). These depositions lead to vacuolization of the cytoplasm, disorganization of the myofibrils, cell hypertrophy, and, finally, fibrosis (Figure). Definitive diagnosis of hydroxychloroquine-induced cardiomyopathy requires the presence of pathognomonic curvilinear bodies3 (these can also be found in neuronal ceroid lipofuscinosis, but without involvement of the myocardium). Myeloid bodies are very suggestive of hydroxychloroquine-induced cardiomyopathy but they are not pathognomonic because they can be present in phospholipidosis induced by cationic amphiphilic drugs (amiodarone, aminoglycosides, and fluoxetine, among others) and also in genetic phospholipidoses (Fabry disease and Niemann-Pick disease). It is therefore necessary to rule out these diseases before relating myeloid bodies to hydroxychloroquine. From the practical point of view, hydroxychloroquine-induced cardiac toxicity should be suspected if ventricular dysfunction develops in a patient who is taking this drug, and should be grounds for discontinuation. Most cases reported in the literature correspond to the restrictive cardiomyopathy phenotype with increased wall thickness as the most common structural finding. The ventricular dilatation phenotype without wall thickening is less frequent. However, in the 3 cases thought to have occurred in our cardiomyopathy unit, presentation was always in the form of dilated cardiomyopathy (Table). The certainty of diagnosis varied from pathognomonic demonstration of curvilinear bodies in endomyocardial biopsy (patient 1) to mere clinical suspicion given the temporal the relationship with drug administration (patient 3). In patient 2, the presence of myeloid bodies was observed without curvilinear ones. Although hydroxychloroquine rarely causes conduction disorders in the electrocardiogram, it can reduce heart rate by a similar mechanism to Ivabradine; consequently, it is beginning to be discussed as a possible antianginal drug.4 There is considerable variability in the minimum dose required to cause cardiac toxicity reported in the literature, and this observation is reflected in the 3 cases described below.
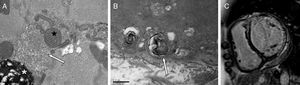
A, Endomyocardial biopsy and electron microscopy for patient 1. Characteristic curvilinear bodies (arrow), along with myelin figures (asterisk, left inferior margin) and abnormal mitochondria (star). B, Endomyocardial biopsy and electron microscopy for patient 2. Myeloid body (arrow), structure formed by concentric layers of electron-dense membranous material surrounded by a single limiting membrane. C, Magnetic resonance imaging, late contrast enhancement sequence, short axis, corresponding to patient 2. Inferolateral transmural fibrosis and linear septal intramural fibrosis can be observed.
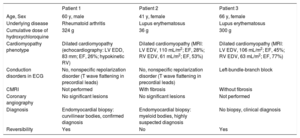
Characteristics of the 3 Patients With Hydroxychloroquine-Induced Cardiomyopathy in the Cardiomyopathy Unit
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age, Sex | 60 y, male | 41 y, female | 66 y, female |
| Underlying disease | Rheumatoid arthritis | Lupus erythematosus | Lupus erythematosus |
| Cumulative dose of hydroxychloroquine | 324 g | 36 g | 300 g |
| Cardiomyopathy phenotype | Dilated cardiomyopathy (echocardiography: LV EDD, 83 mm; EF, 26%; hypokinetic RV) | Dilated cardiomyopathy (MRI: LV EDV, 110 mL/m2; EF, 28%; RV EDV, 61 mL/m2; EF, 53%) | Dilated cardiomyopathy (MRI: LV EDV, 106 mL/m2; EF, 45%; RV EDV, 63 mL/m2; EF, 77%) |
| Conduction disorders in ECG | No, nonspecific repolarization disorder (T wave flattening in precordial leads) | No, nonspecific repolarization disorder (T wave flattening in precordial leads) | Left-bundle-branch block |
| CMRI | Not performed | With fibrosis | Without fibrosis |
| Coronary angiography | No significant lesions | No significant lesions | Not performed |
| Diagnosis | Endomyocardial biopsy: curvilinear bodies, confirmed diagnosis | Endomyocardial biopsy: myeloid bodies, highly suspected diagnosis | No biopsy, clinical diagnosis |
| Reversibility | Yes | No | Yes |
CMRI, cardiac magnetic resonance imaging; ECG, electrocardiogram; EDD, end-diastolic diameter; EDV, end-diastolic volume; EF, ejection fraction; LV, left ventricular; MRI, magnetic resonance imaging; RV, right ventricular.
Patient 1 was a 60-year old man with rheumatoid arthritis (under treatment with methylprednisolone and hydroxychloroquine for 27 months). Echocardiography prior to treatment showed no abnormalities. He presented with heart failure and dilated cardiomyopathy with severely depressed ejection fraction, and no increased wall thickness. His status normalized after treatment discontinuation. Endomyocardial biopsy showed myeloid and curvilinear bodies under electron microscopy, which confirmed the diagnosis of hydroxychloroquine-induced cardiomyopathy.
The second patient was a 41-year-old woman with lupus erythematosus. She initiated treatment with hydroxychloroquine with no renal insufficiency or other history of interest. Three months later, she presented with acute pulmonary edema. Cardiac magnetic resonance imaging showed dilated ventricular volumes with severely depressed ejection fraction, no wall hypertrophy, and late gadolinium enhancement in the inferolateral transmural and septal intramural region. Endomyocardial biopsy showed myeloid bodies but not curvilinear ones. Given the clinical context, the phenotype of cardiomyopathy, and sequencing of the GLA gene, which showed no variants with respect to the reference genome, other phospholipidoses mentioned above were ruled out. Despite withdrawal of the drug, ejection fraction had not improved after 2 years of follow-up.
Patient 3 was a 66-year-old woman with lupus erythematosus and normal echocardiogram prior to initiating therapy who developed dilated cardiomyopathy after 25 months of treatment. The patient presented with exercise-induced dyspnea. Magnetic resonance imaging showed left ventricular dilatation with a slightly depressed ejection fraction, normal wall thickness, and no focal or segmental fibrosis in the late enhancement sequences. The drug was withdrawn and ventricular volumes returned to normal during subsequent follow-up. Hydroxychloroquine-induced cardiomyopathy was therefore suspected clinically. Endomyocardial biopsy was not performed.
These 3 cases of hydroxychloroquine-induced myocardial toxicity highlight the importance of periodic clinical assessment of these patients (even those who have been under treatment for a few months). In the event of minimal clinical suspicion, the use of imaging techniques should be considered to assess whether myocardial involvement is present.
.
We would like to thank Dr Nuria Padullés Zamora, hospital pharmacist, for her collaboration.