The advantages of surgical mitral valve (MV) repair vs MV replacement have been extensively documented and it has become the preferred treatment option for patients with mitral regurgitation. However, recent studies have called into question the durability of MV repair, with a reoperation rate of up to 10% to 15% at 10 years of follow-up.1 In cases of mitral regurgitation recurrence, reoperation often carries a high risk and a significant number of patients do not undergo surgery for this reason.
Isolated case reports have suggested the feasibility of transcatheter MV implantation in the presence of a ring annuloplasty.2 In most of these cases, the transapical approach was used and a Melody® or Edwards SAPIEN percutaneous valve was implanted into the mitral ring.3–6
We report the case of a young man with MV repair failure, in which a percutaneous complete repair of the failed surgery was performed, with transfemoral closure of mitral paravalvular leak and implantation of an Edwards SAPIEN XT valve in the mitral ring in the same procedure.
A 62-year-old man was admitted due to congestive heart failure. Ten years before, the patient underwent coronary artery bypass (left internal mammary to left anterior descending artery and saphenous vein graft to circumflex) with MV repair and implantation of a 30-mm CE Physio semirigid ring (Edwards Lifesciences Inc; Irvine, California, United States). On admission, a transesophageal echocardiogram showed severe mitral regurgitation due to an anteroseptal para-ring leak, with a central intravalvular jet related to leaflet degeneration and moderate left ventricular dysfunction (ejection fraction 40%) with severe pulmonary hypertension. A coronary angiogram was performed ruling out significant coronary disease, with patent coronary grafts. The patient was rejected for a new surgical intervention (logistic EuroSCORE 21.49%, Society of Thoracic Surgeons score 10.23%) and transfemoral valve-in-ring implantation with para-ring leak closure was planned.
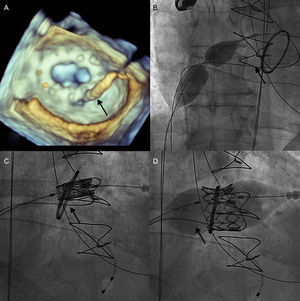
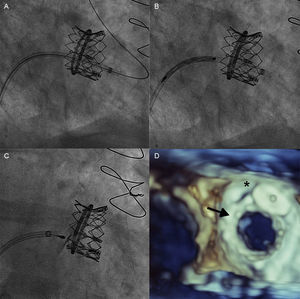
After transeptal puncture, an arteriovenous loop was established with a hydrophilic straight tip wire that had crossed the leak retrograde from the left ventricle, snared in the left atrium and exteriorized through the femoral vein. Subsequently, a left Amplatz catheter was advanced from the venous size into the left atrium, crossing the MV anterograde with a hydrophilic wire, which was captured in the descending aorta and externalized through the femoral artery, creating the second venoarterial loop. After balloon dilation of the septum with a 16-mm balloon, a 29 Edwards SAPIEN XT prosthesis mounted reversely on an 18-F Novaflex delivery catheter (Edwards Lifesciences) was implanted inside the mitral ring under rapid pacing, with slow and controlled balloon inflation (Figure 1). After the valve implant a 7-F sheath was advanced through the first arteriovenous loop and after analysis of the transesophageal echocardiography images, a 14/5 AMPLATZERTM vascular plug III was implanted in the leak with a very good echocardiographic result and minimal residual mitral regurgitation (Figure 2).
The patient was discharged 3 days later and remains asymptomatic 6 months after the procedure.
Complete percutaneous treatment of a failed surgical repair with significant valvular and para-ring regurgitation can be performed in high-risk patients, with transfemoral closure of mitral para-ring leak and implantation of Edwards SAPIEN XT in the mitral ring during the same procedure.
The strategy of the intervention is essential to avoid potential complications, enabling the success of the procedure and improving the final result.
In this case, the 2 arteriovenous loops were performed at the beginning of the procedure, implanting the valve before the leak closure. This approach may have different advantages; the size and shape of the leak can be modified after the valve implantation, providing better apposition between the ring and the surrounding tissue, thus reducing the leak size and easing the para-ring leak closure. In addition, the valve structure provides better anchor for the vascular plug that can be easily delivered, monitoring at the same time a potential interference of the device with the implanted valve.
The first cases of transcatheter valve implantation inside the mitral ring were performed with Melody valves,2 but the use of the Edwards SAPIEN XT is now preferred. The limited size of the Melody® valve restricts the ring size suitable for implantation of this valve. Furthermore, the lower profile and flexing possibilities of the Edwards delivery facilitates valve deployment.
An additional problem for the valve-in-ring implantation is that the mitral rings have a more oval shape whereas the valve has a round shape. In this regard, the greater radial force of the Edwards system valve in addition ti a slow inflation can benefit the adaptation of the ring to the valve shape, with better result.
Complete transfemoral repair of a degenerative surgical mitral bioprosthesis with significant paravalvular regurgitation can be performed in high-risk patients with valve-in-ring implantation and para-ring leak repair during the same procedure.