Minimally invasive cardiac surgery (MICS) has emerged relatively recently for the surgical repair of congenital heart disease (CHD).1–6 We have reported excellent results in terms of surgical outcomes when a MICS approach is employed, and our 20-year experience now comprises more than 1000 cases.1 This includes different surgical approaches, ranging from minimally invasive lower minsternotomies (MS) to right anterior minithoracotomies and right lateral minithoracotomies (RLMT).
The transition process that led us from a full sternotomy to less invasive surgical strategies required improvements in the surgical instrumentation and perfusion strategies to enhance surgical exposure while maintaining a small incision, thus expanding implementation from simple cases, such as an atrial septal defect (ASD) closure, to more complex cases, such as ventricular septal defect (VSD) closure, partial atrioventricular septal defect repair, and partial pulmonary venous return repair.
Our current protocol includes a tailored approach that is based on patient sex and on the underlying pathology. The MS approach was predominantly used at the beginning of our experience and is now reserved mainly for small infants with VSD. A right anterior minithoracotomy was subsequently introduced for the treatment of simple CHD (mainly ASD) in female patients to avoid a midline incision. Most recently, we have moved our incision even more laterally and have introduced the RLMT (figure 1A). This has become our favored approach, as it can be applied for the correction of various CHD while offering excellent outcomes in terms of patient satisfaction and aesthetic results.3
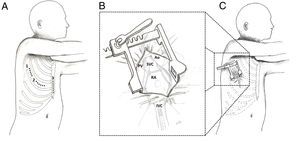
Different accesses for minimally invasive pediatric cardiac surgery. A: graphical representation of the 3 different types of access for minimally invasive cardiac surgery used at the University of Padua. 1, right lateral minithoracotomy. 2, right anterior minithoracotomy. 3, midline lower ministernotomy. B: close-up. C: graphical representation of a right lateral minithoracotomy with the anatomical structures that can be approached through this access. Ao, aorta; IVC, inferior vena cava; PV, pulmonary veins; RA, right atrium; SVC, superior vena cava.
In the RLMT approach, a right thoracotomy of 3 to 4cm is created in the fourth or fifth intercostal space, depending on the main pathology to be addressed (figure 1B,C). The incision is extended from the mid-axillary line toward the anterior axillary line. Peripheral cannulation for cardiopulmonary bypass has been adopted as a standard practice in MICS cases, and currently, our institutional protocol allows a safe femoral arterial cannulation in patients weighing ≥ 15kg and femoral venous cannulation in patients weighing ≥ 7kg. The superior vena cava cannulation can be achieved either directly or percutaneously by cannulating the internal jugular vein.
At present, a total of 219 patients (110 females and 109 males) have undergone surgical correction of CHD by RLMT at our institution. Median age was 7.7 years [interquartile range [IQR, 4.9-13.2 years] and median weight 26.4kg [IQR, 17.3-49.7kg]. The diagnoses requiring surgical repair were ASD in 49% (107/219), partial pulmonary venous return in 25% (55/219), partial atrioventricular septal defect in 12% (27/219), and VSD in 8% (16/219) of the population, respectively. Other diagnoses (6%; 14/219) included aortic valve stenosis or left ventricular outflow obstruction (n=6), inferior sinus venosus ASD (n=6), and mitral valve stenosis (n=2). Peripheral cannulation was used in 94.9% of RLMT cases (208/219). To achieve intracardiac repair, a cross clamp was adopted in 103 patients (47%) and alternatively induced ventricular fibrillation (IVF) in 116 patients (53%). Median CPB, cross clamp and IVF time were 47minutes [IQR, 33-69minutes), 40minutes [IQR, 33-55minutes], and 17minutes [IQR, 13-25 minutes], respectively. The median intensive care unit (ICU) and hospital length of stay were 1 day [IQR, 1-1 day] and 4 days [IQR, 4-5.5 days], respectively. Blood transfusion was required in 8% (18/219) of the patients. The major complications rate was 2.7% (6/219) and comprised postoperative bleeding requiring reoperation (3/6) and vascular access complications (3/6). None of the patients in this group required conversion to traditional median sternotomy.
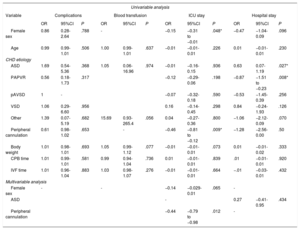
Logistic and linear regression models were used to identify risk factors for postoperative complications, blood transfusion requirement, and ICU or hospital stay. Peripheral cannulation was significantly associated with a shorter ICU stay in the multivariable analysis (coefficient: −0.44; 95% confidence interval, −0.79 to −0.98, P=.012) (table 1). None of the underlying treated CHD was associated with an increased risk of postoperative complications.
Univariable and multivariable regression analysis of predictors for complications, need for transfusion, ICU and length of stay
| Univariable analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Complications | Blood transfusion | ICU stay | Hospital stay | ||||||||
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Female sex | 0.86 | 0.28-2.64 | .788 | - | −0.15 | −0.31 to −0.01 | .048* | −0.47 | −1.04-0.09 | .096 | ||
| Age | 0.99 | 0.99-1.01 | .506 | 1.00 | 0.99-1.01 | .637 | −0.01 | −0.01-0.01 | .226 | 0.01 | −0.01-0.01 | .230 |
| CHD etiology | ||||||||||||
| ASD | 1.69 | 0.54-5.36 | .368 | 1.05 | 0.06-16.96 | .974 | −0.01 | −0.16-0.15 | .936 | 0.63 | 0.07-1.19 | .027* |
| PAPVR | 0.56 | 0.18-1.73 | .317 | −0.12 | −0.29-0.06 | .198 | −0.87 | −1.51 to −0.23 | .008* | |||
| pAVSD | 1 | - | −0.07 | −0.32-0.18 | .590 | −0.53 | −1.45-0.39 | .256 | ||||
| VSD | 1.06 | 0.29-6.60 | .956 | 0.16 | −0.14-0.45 | .298 | 0.84 | −0.24-1.93 | .126 | |||
| Other | 1.39 | 0.07-5.19 | .682 | 15.69 | 0.93-265.4 | .056 | 0.04 | −0.27-0.36 | .800 | −1.06 | −2.12-0.09 | .070 |
| Peripheral cannulation | 0.61 | 0.98-1.02 | .653 | - | −0.46 | −0.81 to −0.12 | .009* | −1.28 | −2.56-0.00 | .50 | ||
| Body weight | 1.01 | 0.98-1.01 | .693 | 1.05 | 0.99-1.12 | .077 | −0.01 | −0.01-0.01 | .073 | 0.01 | −0.01-0.02 | .333 |
| CPB time | 1.01 | 0.99-1.01 | .581 | 0.99 | 0.94-1.04 | .736 | 0.01 | −0.01-0.01 | .839 | .01 | −0.01-0.01 | .920 |
| IVF time | 1.01 | 0.96-1.04 | .883 | 1.03 | 0.98-1.07 | .276 | −0.01 | −0.01-0.01 | .664 | −.01 | −0.03-0.01 | .432 |
| Multivariable analysis | ||||||||||||
| Female sex | - | - | −0.14 | −0.029-0.01 | .065 | - | ||||||
| ASD | - | 0.27 | −0.41-0.95 | .434 | ||||||||
| Peripheral cannulation | −0.44 | −0.79 to −0.98 | .012 | - | ||||||||
95%CI, 95% confidence interval; ASD, atrial septal defect; CHD, congenital heart defect; CPB, cardiopulmonary bypass; ICU, intensive care unit; IVF, induced ventricular fibrillation; OR, odds ratio PAPVR, partial anomalous pulmonary venous return; pAVSD; partial atrioventricular septal defect; VSD, ventricular septal defect.
We began regularly using RLMT as a MICS strategy in 2013. In addition to comparable outcomes compared with median sternotomy and other approaches, we have also shown that in long term follow-up, the vast majority of patients treated with MICS were satisfied with the cosmetic result, and satisfaction was higher in patients undergoing RLMT or right anterior minithoracotomy than in patients with MS. While other centers have incorporated the use of RLMT for ASD repair,3,5 we have successfully expanded the application of this strategy to numerous diagnoses of varying complexity without sacrificing clinical outcomes.1 The lateralization process of our surgical approach has consequently evolved successfully over time.
Peripheral cannulation was associated with a shorter ICU stay. Early in our MICS experience, peripheral cannulation was used selectively for patients with a body weight greater than 30 kg, largely due to the anatomic limitations of the femoral vessels in smaller patients. We have since expanded the use of peripheral cannulation as stated above.
While MICS is able to reduce postoperative morbidity, this would not be possible without a coordinated multidisciplinary approach among all members of the operative and postoperative team.2 Surgical outcomes for CHD are continuously improving, and MICS is currently emerging as the next developmental phase to maintain excellent outcomes while reducing the psychological and physical trauma of surgery, aspects that are particularly important to pediatric patients. Morbidity and mortality will certainly continue to be the most important outcomes for patients with CHD. However, the potential for emerging strategies, such as MICS via a RLMT to reduce postoperative trauma, and improve secondary considerations such as aesthetic outcomes, while maintaining quality standards, will play an increasingly important role in the implementation of cardiac surgery in the future.