Traditionally, infective endocarditis (IE) has been considered as a disease affecting patients with underlying heart disease (HD).1 This profile appears to have changed in recent decades, in that EI affects both patients with degenerative valve disease and those with no apparent HD.1–4 Various studies have shown that the proportion of patients with IE and no underlying HD has increased in our setting.2,5,6 Although each type of IE appears to have distinct epidemiologic and prognostic characteristics,2,5 it is not known whether the profile of non-HDIE has changed in recent years, which could have implications for prognosis. The objectives of our study were: a) to compare the characteristics of HD-associated left-sided native-valve IE (HDIE) and non-HD-associated left-sided native-valve IE (non-HDIE) diagnosed at our center between 1987 and 2013, and b) to study changes in the profile of non-HDIE during this period.
We analyzed a series of 420 consecutive patients diagnosed with IE between 1987 and 2013, of which 240 (57%) had left-sided native-valve IE. Diagnosis was made according to the Von Reyn, Duke and modified Duke criteria, depending on the time period. The management protocol did not change over this period, except for the introduction of transesophageal echocardiography during the 1990s. Each patient was classified as having either HDIE or non-HDIE, depending on the results of transthoracic and transesophageal echocardiography during the episode of IE, previous echocardiograms, medical history, and surgical and autopsy findings. The valve was considered normal when the portions of the leaflets that were unaffected by infection were normal and there was no chordal involvement or commissural fusion.5 The active phase of the disease was defined as the first 6 weeks from symptom onset. Urgent surgery was defined as that which could not be postponed for more than 24hours without risk to the patient's life, while elective surgery was defined as that carried out after 24hours.
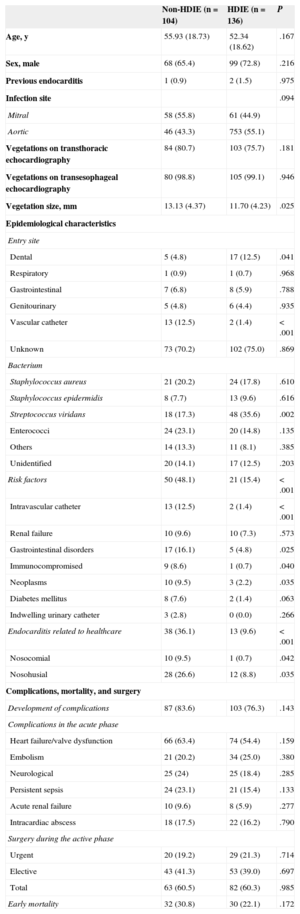
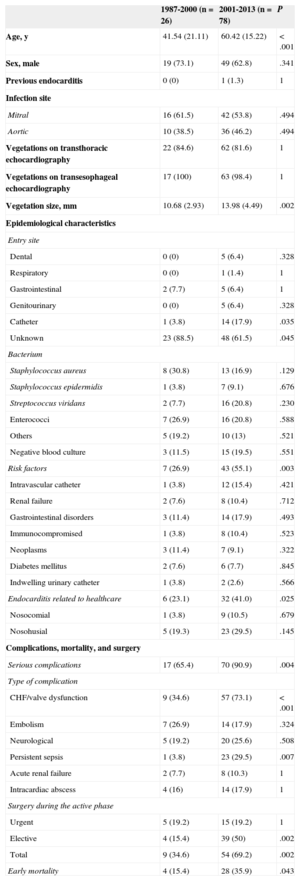
Of the 240 cases of left native-valve IE, 104 (43%) were classified as non-HDIE, and the remaining 136 (57%) were diagnosed with HDIE. The proportion of cases of non-HDIE increased significantly, constituting 25.7% of cases of left-sided native-vale IE from 1987 to 2000 and 56.1% from 2001 to 2013 (P<.001). The characteristics of both types of patients during the entire 27-year period are shown in Table 1. The rate of serious complications, premature mortality, and need for surgery were similar, whereas there were significant differences in epidemiological characteristics: patients with non-HDIE had a higher prevalence of non-cardiac risk factors and predisposing comorbidities (chronic gastrointestinal diseases, malignancies, renal failure, diabetes, immunosuppression) and healthcare-related procedures (intravascular catheters, and nosocomial and nosohusial EI), but less frequently had IE caused by Streptococcus viridans (Table 1). The characteristics of patients with non-HDIE from 1987 to 2000 and from 2001 to 2013 are shown in Table 2, highlighting significant changes in both the clinical and epidemiological profile between the 2 periods. In the most recent period, patients with non-HDIE were much older (almost 20 years older, on average), had larger vegetations, a tendency to have IE caused less by Staphylococcus aureus and more by Streptococcus viridans, and a higher prevalence of non-cardiac risk factors for IE, and more frequently had IE associated with health care procedures. The incidence of serious complications during the active phase of IE, especially of heart failure/valve dysfunction and persistent sepsis, was also significantly higher during the most recent period. Early mortality more than doubled in the second period (35.9% vs 15.4%; P=.043), as did the need for early surgery (69.2% vs 34.6%; P=.002) (Table 2).
Comparative Characteristics Between Left-sided Native-valve Endocarditis With and Without Underlying Heart Disease (n=240)
| Non-HDIE (n=104) | HDIE (n=136) | P | |
|---|---|---|---|
| Age, y | 55.93 (18.73) | 52.34 (18.62) | .167 |
| Sex, male | 68 (65.4) | 99 (72.8) | .216 |
| Previous endocarditis | 1 (0.9) | 2 (1.5) | .975 |
| Infection site | .094 | ||
| Mitral | 58 (55.8) | 61 (44.9) | |
| Aortic | 46 (43.3) | 753 (55.1) | |
| Vegetations on transthoracic echocardiography | 84 (80.7) | 103 (75.7) | .181 |
| Vegetations on transesophageal echocardiography | 80 (98.8) | 105 (99.1) | .946 |
| Vegetation size, mm | 13.13 (4.37) | 11.70 (4.23) | .025 |
| Epidemiological characteristics | |||
| Entry site | |||
| Dental | 5 (4.8) | 17 (12.5) | .041 |
| Respiratory | 1 (0.9) | 1 (0.7) | .968 |
| Gastrointestinal | 7 (6.8) | 8 (5.9) | .788 |
| Genitourinary | 5 (4.8) | 6 (4.4) | .935 |
| Vascular catheter | 13 (12.5) | 2 (1.4) | <.001 |
| Unknown | 73 (70.2) | 102 (75.0) | .869 |
| Bacterium | |||
| Staphylococcus aureus | 21 (20.2) | 24 (17.8) | .610 |
| Staphylococcus epidermidis | 8 (7.7) | 13 (9.6) | .616 |
| Streptococcus viridans | 18 (17.3) | 48 (35.6) | .002 |
| Enterococci | 24 (23.1) | 20 (14.8) | .135 |
| Others | 14 (13.3) | 11 (8.1) | .385 |
| Unidentified | 20 (14.1) | 17 (12.5) | .203 |
| Risk factors | 50 (48.1) | 21 (15.4) | <.001 |
| Intravascular catheter | 13 (12.5) | 2 (1.4) | <.001 |
| Renal failure | 10 (9.6) | 10 (7.3) | .573 |
| Gastrointestinal disorders | 17 (16.1) | 5 (4.8) | .025 |
| Immunocompromised | 9 (8.6) | 1 (0.7) | .040 |
| Neoplasms | 10 (9.5) | 3 (2.2) | .035 |
| Diabetes mellitus | 8 (7.6) | 2 (1.4) | .063 |
| Indwelling urinary catheter | 3 (2.8) | 0 (0.0) | .266 |
| Endocarditis related to healthcare | 38 (36.1) | 13 (9.6) | <.001 |
| Nosocomial | 10 (9.5) | 1 (0.7) | .042 |
| Nosohusial | 28 (26.6) | 12 (8.8) | .035 |
| Complications, mortality, and surgery | |||
| Development of complications | 87 (83.6) | 103 (76.3) | .143 |
| Complications in the acute phase | |||
| Heart failure/valve dysfunction | 66 (63.4) | 74 (54.4) | .159 |
| Embolism | 21 (20.2) | 34 (25.0) | .380 |
| Neurological | 25 (24) | 25 (18.4) | .285 |
| Persistent sepsis | 24 (23.1) | 21 (15.4) | .133 |
| Acute renal failure | 10 (9.6) | 8 (5.9) | .277 |
| Intracardiac abscess | 18 (17.5) | 22 (16.2) | .790 |
| Surgery during the active phase | |||
| Urgent | 20 (19.2) | 29 (21.3) | .714 |
| Elective | 43 (41.3) | 53 (39.0) | .697 |
| Total | 63 (60.5) | 82 (60.3) | .985 |
| Early mortality | 32 (30.8) | 30 (22.1) | .172 |
HDEI, heart disease-associated infective endocarditis; non-HDIE, non-heart disease-associated infective endocarditis.
Data are expressed as mean (standard deviation).
Comparison of the Characteristics of the Subgroup of Patients With Endocarditis Without Underlying Heart Disease Between the Periods 1987 to 2000 and 2001 to 2013 (n=104)
| 1987-2000 (n=26) | 2001-2013 (n=78) | P | |
|---|---|---|---|
| Age, y | 41.54 (21.11) | 60.42 (15.22) | <.001 |
| Sex, male | 19 (73.1) | 49 (62.8) | .341 |
| Previous endocarditis | 0 (0) | 1 (1.3) | 1 |
| Infection site | |||
| Mitral | 16 (61.5) | 42 (53.8) | .494 |
| Aortic | 10 (38.5) | 36 (46.2) | .494 |
| Vegetations on transthoracic echocardiography | 22 (84.6) | 62 (81.6) | 1 |
| Vegetations on transesophageal echocardiography | 17 (100) | 63 (98.4) | 1 |
| Vegetation size, mm | 10.68 (2.93) | 13.98 (4.49) | .002 |
| Epidemiological characteristics | |||
| Entry site | |||
| Dental | 0 (0) | 5 (6.4) | .328 |
| Respiratory | 0 (0) | 1 (1.4) | 1 |
| Gastrointestinal | 2 (7.7) | 5 (6.4) | 1 |
| Genitourinary | 0 (0) | 5 (6.4) | .328 |
| Catheter | 1 (3.8) | 14 (17.9) | .035 |
| Unknown | 23 (88.5) | 48 (61.5) | .045 |
| Bacterium | |||
| Staphylococcus aureus | 8 (30.8) | 13 (16.9) | .129 |
| Staphylococcus epidermidis | 1 (3.8) | 7 (9.1) | .676 |
| Streptococcus viridans | 2 (7.7) | 16 (20.8) | .230 |
| Enterococci | 7 (26.9) | 16 (20.8) | .588 |
| Others | 5 (19.2) | 10 (13) | .521 |
| Negative blood culture | 3 (11.5) | 15 (19.5) | .551 |
| Risk factors | 7 (26.9) | 43 (55.1) | .003 |
| Intravascular catheter | 1 (3.8) | 12 (15.4) | .421 |
| Renal failure | 2 (7.6) | 8 (10.4) | .712 |
| Gastrointestinal disorders | 3 (11.4) | 14 (17.9) | .493 |
| Immunocompromised | 1 (3.8) | 8 (10.4) | .523 |
| Neoplasms | 3 (11.4) | 7 (9.1) | .322 |
| Diabetes mellitus | 2 (7.6) | 6 (7.7) | .845 |
| Indwelling urinary catheter | 1 (3.8) | 2 (2.6) | .566 |
| Endocarditis related to healthcare | 6 (23.1) | 32 (41.0) | .025 |
| Nosocomial | 1 (3.8) | 9 (10.5) | .679 |
| Nosohusial | 5 (19.3) | 23 (29.5) | .145 |
| Complications, mortality, and surgery | |||
| Serious complications | 17 (65.4) | 70 (90.9) | .004 |
| Type of complication | |||
| CHF/valve dysfunction | 9 (34.6) | 57 (73.1) | <.001 |
| Embolism | 7 (26.9) | 14 (17.9) | .324 |
| Neurological | 5 (19.2) | 20 (25.6) | .508 |
| Persistent sepsis | 1 (3.8) | 23 (29.5) | .007 |
| Acute renal failure | 2 (7.7) | 8 (10.3) | 1 |
| Intracardiac abscess | 4 (16) | 14 (17.9) | 1 |
| Surgery during the active phase | |||
| Urgent | 5 (19.2) | 15 (19.2) | 1 |
| Elective | 4 (15.4) | 39 (50) | .002 |
| Total | 9 (34.6) | 54 (69.2) | .002 |
| Early mortality | 4 (15.4) | 28 (35.9) | .043 |
CHF, chronic heart failure.
Data are expressed as mean (standard deviation).
Our data indicate that in our setting non-HDIE has shifted during the last 25 years toward a more serious clinical and prognostic profile (higher incidence of serious complications, need for surgery, and early mortality). This change may be because non-HDIE patients in the most recent period were much older and had a higher prevalence of severe comorbidities and non-cardiac risk factors for IE (chronic gastrointestinal and kidney diseases, immunosuppression, catheters and long-term vascular access). This type of IE now represents more than half of cases of native-valve IE,6 which may partly explain why the clinical characteristics, morbidity and mortality of non-HDIE are increasingly similar to those of HDIE, as shown in Table 1. This change also obliges us to change our attitude toward non-HDIE, which is no longer a more “benign” disease than HDIE. Infective endocarditis without predisposing HD should be suspected in the absence of predisposing cardiac disease to allow its early diagnosis and treatment, thus helping to reduce its increasing mortality.