Patients with atrial fibrillation often have numerous concurrent diseases that are associated with a worse prognosis. Although several studies have addressed the causes of death in these patients, only limited data are available from clinical practice for patients under treatment with direct-acting oral anticoagulants (DAOAs). Given that patients who receive these agents in Spain usually have a different clinical profile to those who receive vitamin K antagonists,2,3 we evaluated the causes and predictors of death in patients with nonvalvular atrial fibrillation (NVAF) who started treatment with DAOAs in 3 Spanish health areas. For our study, we included 973 consecutive patients with NVAF who were prescribed DAOA for the first time between January 2013 and December 2014. Patients were selected from the prescription database, which includes information from all medical prescriptions of all the health areas included in the study, given that electronic prescription is mandatory. Subsequently, all electronic medical records were reviewed to check whether NVAF was present. We excluded patients with an indication for temporary anticoagulation and indications other than atrial fibrillation; patients with hypertrophic cardiomyopathy, moderate/severe rheumatic mitral stenosis, or mechanical valve prosthesis; and those who had already taken DAOAs. Patients were followed up from the date of prescription through to a common end date. The primary outcome measure was death. Information on this outcome was available for 99.8% of the patients. Deaths were reported by the clinical cardiologist, using a standard form that included a structured description of the date and place of death, circumstances in which death occurred, treatments administered, etc. The information was obtained through electronic chart review, death certificates, reports of out-of-hospital emergency personnel, and telephone contact with the family in the event of death. To identify the factors associated with death, a multivariate Cox regression analysis was undertaken, which was used to calculate the hazard ratio. SPSS v21 and STATA v13 statistical packages were used for the statistical analysis. The present study was approved by the ethics committees of the participating centers.
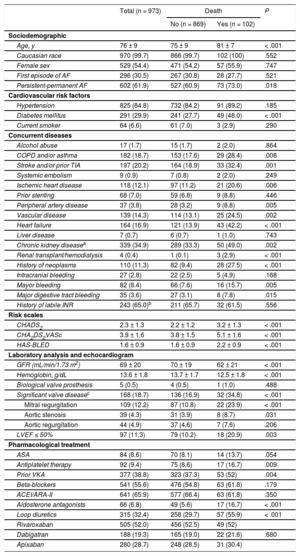
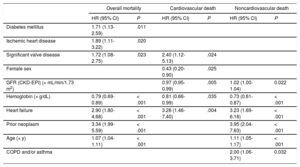
The characteristics of the study population are summarized in Table 1. For a mean follow-up of 646 days (range, 470-839 days), 102 deaths were recorded (5.85/100 patient-years), of which 34 were of cardiac cause (1.95/100 patient-years), 55 of noncardiac cause (3.16/100 patient-years), and 13 of undetermined cause (0.74/100 patient-years). Table 1 of the supplementary material shows the incidence of the causes of death in the study population. The most frequent causes of death were neoplasms (1.20/100 patient-years), infections (0.92/100 patient-years), and heart failure (0.80 patient-years). Patients who died were older and had more concurrent diseases and higher scores on the risk scales for thromboembolic and bleeding events (Table 1). The predictors of overall mortality, whether of cardiovascular origin or not, are shown in Table 2. The C-statistic of the model was 0.82 for overall mortality (95% confidence interval [CI], 0.77-0.87; P < .001), 0.81 for cardiovascular mortality (95% CI, 0.73-0.89; P < .001), and 0.81 for noncardiovascular mortality (95% CI, 0.74-0.87; P < .001) (Hosmer-Lemeshow test, P > .05 in the 3 models). During follow-up, mortality was similar regardless of the type of anticoagulant or cause of death (Tables 2 and 3 of the supplementary material).
Baseline Characteristics of the Study Population by Survival
| Total (n = 973) | Death | P | ||
|---|---|---|---|---|
| No (n = 869) | Yes (n = 102) | |||
| Sociodemographic | ||||
| Age, y | 76 ± 9 | 75 ± 9 | 81 ± 7 | < .001 |
| Caucasian race | 970 (99.7) | 866 (99.7) | 102 (100) | .552 |
| Female sex | 529 (54.4) | 471 (54.2) | 57 (55.9) | .747 |
| First episode of AF | 296 (30.5) | 267 (30.8) | 28 (27.7) | .521 |
| Persistent-permanent AF | 602 (61.9) | 527 (60.9) | 73 (73.0) | .018 |
| Cardiovascular risk factors | ||||
| Hypertension | 825 (84.8) | 732 (84.2) | 91 (89.2) | .185 |
| Diabetes mellitus | 291 (29.9) | 241 (27.7) | 49 (48.0) | < .001 |
| Current smoker | 64 (6.6) | 61 (7.0) | 3 (2.9) | .290 |
| Concurrent diseases | ||||
| Alcohol abuse | 17 (1.7) | 15 (1.7) | 2 (2.0) | .864 |
| COPD and/or asthma | 182 (18.7) | 153 (17.6) | 29 (28.4) | .008 |
| Stroke and/or prior TIA | 197 (20.2) | 164 (18.9) | 33 (32.4) | .001 |
| Systemic embolism | 9 (0.9) | 7 (0.8) | 2 (2.0) | .249 |
| Ischemic heart disease | 118 (12.1) | 97 (11.2) | 21 (20.6) | .006 |
| Prior stenting | 68 (7.0) | 59 (6.8) | 9 (8.8) | .446 |
| Peripheral artery disease | 37 (3.8) | 28 (3.2) | 9 (8.8) | .005 |
| Vascular disease | 139 (14.3) | 114 (13.1) | 25 (24.5) | .002 |
| Heart failure | 164 (16.9) | 121 (13.9) | 43 (42.2) | < .001 |
| Liver disease | 7 (0.7) | 6 (0.7) | 1 (1.0) | .743 |
| Chronic kidney diseasea | 339 (34.9) | 289 (33.3) | 50 (49.0) | .002 |
| Renal transplant/hemodialysis | 4 (0.4) | 1 (0.1) | 3 (2.9) | < .001 |
| History of neoplasms | 110 (11.3) | 82 (9.4) | 28 (27.5) | < .001 |
| Intracranial bleeding | 27 (2.8) | 22 (2.5) | 5 (4.9) | .168 |
| Mayor bleeding | 82 (8.4) | 66 (7.6) | 16 (15.7) | .005 |
| Major digestive tract bleeding | 35 (3.6) | 27 (3.1) | 8 (7.8) | .015 |
| History of labile INR | 243 (65.0)b | 211 (65.7) | 32 (61.5) | .556 |
| Risk scales | ||||
| CHADS2 | 2.3 ± 1.3 | 2.2 ± 1.2 | 3.2 ± 1.3 | < .001 |
| CHA2DS2VASc | 3.9 ± 1.6 | 3.8 ± 1.5 | 5.1 ± 1.6 | < .001 |
| HAS-BLED | 1.6 ± 0.9 | 1.6 ± 0.9 | 2.2 ± 0.9 | < .001 |
| Laboratory analysis and echocardiogram | ||||
| GFR (mL/min/1.73 m2) | 69 ± 20 | 70 ± 19 | 62 ± 21 | < .001 |
| Hemoglobin, g/dL | 13.6 ± 1.8 | 13.7 ± 1.7 | 12.5 ± 1.8 | < .001 |
| Biological valve prosthesis | 5 (0.5) | 4 (0.5) | 1 (1.0) | .488 |
| Significant valve diseasec | 168 (18.7) | 136 (16.9) | 32 (34.8) | < .001 |
| Mitral regurgitation | 109 (12.2) | 87 (10.8) | 22 (23.9) | < .001 |
| Aortic stenosis | 39 (4.3) | 31 (3.9) | 8 (8.7) | .031 |
| Aortic regurgitation | 44 (4.9) | 37 (4.6) | 7 (7.6) | .206 |
| LVEF ≤ 50% | 97 (11.3) | 79 (10.2) | 18 (20.9) | .003 |
| Pharmacological treatment | ||||
| ASA | 84 (8.6) | 70 (8.1) | 14 (13.7) | .054 |
| Antiplatelet therapy | 92 (9.4) | 75 (8.6) | 17 (16.7) | .009 |
| Prior VKA | 377 (38.8) | 323 (37.3) | 53 (52) | .004 |
| Beta-blockers | 541 (55.6) | 476 (54.8) | 63 (61.8) | .179 |
| ACEI/ARA-II | 641 (65.9) | 577 (66.4) | 63 (61.8) | .350 |
| Aldosterone antagonists | 66 (6.8) | 49 (5.6) | 17 (16.7) | < .001 |
| Loop diuretics | 315 (32.4) | 258 (29.7) | 57 (55.9) | < .001 |
| Rivaroxaban | 505 (52.0) | 456 (52.5) | 49 (52) | |
| Dabigatran | 188 (19.3) | 165 (19.0) | 22 (21.6) | .680 |
| Apixaban | 280 (28.7) | 248 (28.5) | 31 (30.4) | |
ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ASA, acetylsalicylic acid; ARA-II, angiotensin II receptor antagonist; CHADS2, Heart failure, hypertension, age > 90 years, diabetes mellitus, and Stroke (double); CHA2DS2VASc, Heart failure, hypertension, age ≥ 65 years [double], diabetes mellitus, stroke (double); CKD-EP, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HAS-BLED, hypertension, abnormal renal/liver function, stroke, history or predisposition to bleeding, INR lability, age > 65 years, and concomitant medication or alcohol; INR, international normalized ratio; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Data expressed as mean ± SD or No. (%).
Multivariate Analysis of Cox Proportional Risks for Prediction of Overall, Cardiovascular, and Noncardivascular Death
| Overall mortality | Cardiovascular death | Noncardiovascular death | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Diabetes mellitus | 1.71 (1.13-2.59) | .011 | ||||
| Ischemic heart disease | 1.89 (1.11-3.22) | .020 | ||||
| Significant valve disease | 1.72 (1.08-2.75) | .023 | 2.40 (1.12-5.13) | .024 | ||
| Female sex | 0.43 (0.20-0.90) | .025 | ||||
| GFR (CKD-EPI) (× mL/min/1.73 m2) | 0.97 (0.95-0.99) | .005 | 1.02 (1.00-1.04) | 0.022 | ||
| Hemoglobin (× g/dL) | 0.79 (0.69-0.89) | < .001 | 0.81 (0.66-0.99) | .035 | 0.73 (0.61-0.87) | < .001 |
| Heart failure | 2.90 (1.80-4.68) | < .001 | 3.28 (1.46-7.40) | .004 | 3.23 (1.69-6.18) | < .001 |
| Prior neoplasm | 3.34 (1.99-5.59) | < .001 | 3.95 (2.04-7.63) | < .001 | ||
| Age (× y) | 1.07 (1.04-1.11) | < .001 | 1.11 (1.05-1.17) | < .001 | ||
| COPD and/or asthma | 2.00 (1.06-3.71) | 0.032 | ||||
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HR, hazard ratio; 95% CI, 95% confidence interval; TIA, transient ischemic attack.
Multivariate model adjusted for age, sex, persistent-permanent AF, diabetes mellitus, COPD and/or asthma, stroke and/or prior TIA, ischemic heart disease, peripheral artery disease, heart failure, neoplasm, previous major bleeding, GFR estimated according to CKD-EPI, hemoglobin, significant valve disease, left ventricular ejection fraction, and direct-acting oral anticoagulant received.
This study is the first to assess the causes of death in a recent and multicenter cohort of patients with NVAF who started treatment with DAOA. Our study shows high mortality in this type of patient, and the most frequent causes of death were neoplasms, infections, and heart failure, whereas deaths due to embolic and/or bleeding events were infrequent. In contrast, the main cause of death in pivotal clinical trials was of cardiovascular origin,1–3 which may reflect differences between the clinical profile of the patients included in clinical trials and those who receive DAOAs in daily clinical practice. We therefore believe it is useful to study the causes of death and identify specific predictors for death in these patients in order to improve planning of strategies to increase survival. As shown above, this study identified as predictors risk markers such as age and sex on the one hand and modifiable risk factors such as diabetes mellitus, ischemic heart disease, heart failure, neoplasms, renal failure, anemia, chronic obstructive pulmonary disease, and valve diseases, on the other. These modifiable risk factors can be targeted with preventive and therapeutic measures. Therefore, the main objectives should be to improve cardiovascular risk factor control and achieve adherence to the recommendations of clinical practice guidelines for the management of concurrent diseases associated with atrial fibrillation.4 Finally, the main limitations of this study are its retrospective design and the absence of a control group, as they prevent us from knowing for certain whether the observed increased mortality occurs in all patients with NVAF regardless of the anticoagulant prescribed or whether DAOA therapy somehow selects patients at higher risk.