Hypertension is an important risk factor for myocardial infarction, heart failure, and stroke. Blood pressure (BP) control in hypertensive patients is of prime importance but is achieved in too few patients, despite the availability of multiple safe and effective therapeutic strategies1. Therefore, the introduction of new strategies to improve and optimize BP control in hypertensive patients is an unmet need.
The identification of new biomarkers with proven predictive value in estimating cardiovascular risk (CVR), such as genetic variants recently identified in genome-wide association studies, allow better stratification of CVR estimation when formulated as a multi-locus genetic risk score (GRS)2 even related to subclinical atherosclerosis3. The application of a GRS, based on genetic variants associated with coronary heart disease and independent of classical risk factors, in participants from 2 cohort studies, the REGICOR study and the Framingham Heart Study, improved in risk classification, particularly in patients at intermediary coronary risk4.
Considering that most coronary events occur in individuals classified as having a low or intermediate risk using traditional CVR scores, we hypothesized that the incorporation of GRS in the clinical evaluation could help improve individual patient's perception of the real CVR, and that this awareness would provide a greater stimulus to achieve both adherence to antihypertensive therapy and lifestyle changes, leading to better BP control. The objective of this pilot study was to test the hypothesis that knowledge of the GRS in uncontrolled hypertensive patients would improve BP control, assessed by 24-hour ambulatory blood pressure monitoring (ABPM).
We carried out a 16-week prospective, randomized, single-blind cohort study in 2 parallel groups. We included 80 consecutive patients with uncontrolled ambulatory BP (24 hour-ABPM ≥ 130/80mmHg), who were randomized 1:1 to 2 groups: a) the GRS group, who underwent an additional genetic test (Cardio inCode Score, Ferrer inCode, Barcelona, Spain) to determine the presence of genetic variants that improve CVR estimation (GRS group) and b) the control group who received usual care. Baseline CVR according to REGICOR function was calculated5. At baseline and after 16 weeks, 24 hour-ABPM was performed. The investigators were blinded to the genetic risk when making examinations and therapeutic adjustments.
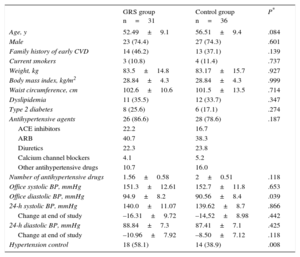
Of the 80 patients randomized, 31 patients in the GRS group and 36 controls were finally analyzed. The baseline characteristics of the 67 patients who completed the study were well balanced between the 2 groups (Table 1). The mean age was 54.5±9.3 years, 74.3% were male, and the proportion of diabetes and dyslipidemia was similar. More than 80% of patients were taking antihypertensive medication in the 3 months before study entry, without significant between-group differences (intervention group 83.6% vs control group 78.6%).
Baseline Characteristics of Participants Who Completed the Study
| GRS group n=31 | Control group n=36 | P* | |
|---|---|---|---|
| Age, y | 52.49±9.1 | 56.51±9.4 | .084 |
| Male | 23 (74.4) | 27 (74.3) | .601 |
| Family history of early CVD | 14 (46.2) | 13 (37.1) | .139 |
| Current smokers | 3 (10.8) | 4 (11.4) | .737 |
| Weight, kg | 83.5±14.8 | 83.17±15.7 | .927 |
| Body mass index, kg/m2 | 28.84±4.3 | 28.84±4.3 | .999 |
| Waist circumference, cm | 102.6±10.6 | 101.5±13.5 | .714 |
| Dyslipidemia | 11 (35.5) | 12 (33.7) | .347 |
| Type 2 diabetes | 8 (25.6) | 6 (17.1) | .274 |
| Antihypertensive agents | 26 (86.6) | 28 (78.6) | .187 |
| ACE inhibitors | 22.2 | 16.7 | |
| ARB | 40.7 | 38.3 | |
| Diuretics | 22.3 | 23.8 | |
| Calcium channel blockers | 4.1 | 5.2 | |
| Other antihypertensive drugs | 10.7 | 16.0 | |
| Number of antihypertensive drugs | 1.56±0.58 | 2±0.51 | .118 |
| Office systolic BP, mmHg | 151.3±12.61 | 152.7±11.8 | .653 |
| Office diastolic BP, mmHg | 94.9±8.2 | 90.56±8.4 | .039 |
| 24-h systolic BP, mmHg | 140.0±11.07 | 139.62±8.7 | .866 |
| Change at end of study | –16.31±9.72 | –14,52±8.98 | .442 |
| 24-h diastolic BP, mmHg | 88.84±7.3 | 87.41±7.1 | .425 |
| Change at end of study | –10.96±7.92 | –8.50±7.12 | .118 |
| Hypertension control | 18 (58.1) | 14 (38.9) | .008 |
ACE inhibitors, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; BP, blood pressure; CVD, cardiovascular disease; GRS, genetic risk score.
Unless otherwise indicated, data are expressed as n (%) or mean±standard deviation.
No significant differences were observed in the baseline distribution according to the individual CVR based on the 10-year predicted risk of coronary heart disease. Less than 10% of patients had an intermediate-high or high CVR according to the REGICOR scale, 27% were at intermediate-low risk and 65% had low CVR. Table 2 shows the reclassification of CVR based on the 10-year predicted risk of coronary heart disease, with and without adding the GRS. Eleven (35%) patients were reclassified into a higher risk group and 1 patient was reclassified into a lower risk group with respect to the baseline REGICOR scale.
Reclassification According to the 10-year Predicted Risk of Coronary Heart Disease With and Without the Genetic Risk Score
Between-group baseline 24 hour-ABPM values were similar, without differences in daytime and night-time periods. The adjusted changes from baseline were -16.31 (95% confidence interval [95% CI], -6.61 to -26.01) for mean 24 hour-SBP in the GRS group and -14.52 (95%CI, -5.62 to -23.42) mmHg in the control group (P=.442), without differences in therapeutic adjustments between groups (Table of the supplementary material). Hypertension control rates defined by ABPM standard cutoffs improved more in GRS patients than in controls (58.1% vs 38.9%, P=.008).
When ABPM control was analyzed by taking into account the CVR score according to GRS information, BP control by ABPM criteria was achieved in all patients assigned to a high CVR and in 75% of those assigned to an intermediate-high CVR but was achieved in only 66% of intermediate-high risk patients assigned to the control group. Only 40% of patients with an intermediate-low CVR and 42% of those with a low CVR achieved normal ABPM values at the end of study, without significant differences between the 2 groups. No differences were observed in antihypertensive therapy between the 2 groups at the end of study [number of antihypertensive drugs: 2.0 (95%CI, 1.52-2.48) in the GRS group vs 2.33 (95%CI, 1.8-2.86) in the control group (P=.142)].
The results of this pilot study showed that patient knowledge of the impact of genetics on their CVR improves hypertension control rates defined by ABPM standard cutoffs (24 hour-ABPM<130/80mmHg), especially in patients at high and very-high CVR. It is impossible to determine whether the greater BP lowering observed and the higher rate of BP control in the GRS group was a consequence of genetic risk knowledge per se or simply of greater awareness of their overall CVR. What is important is that better knowledge of the risk lead to improved BP control, probably secondary to increased adherence to antihypertensive therapy. Larger studies are required to corroborate these results and strengthen the importance of raising awareness of personal CVR to improve BP control and reduce morbidity and mortality in patients with hypertension.
CONFLICTS OF INTERESTThis works was supported by Ferrer. E. Salas was a member of Gendiag.exe, S.L. and an owner of a license of Cardio In Core Score. R. Elosua and J. Marrugat have received payments from Ferrer inCode.