The current guidelines from the European Society of Cardiology for the treatment of cardiogenic shock (CS) put circulatory support with venoarterial extracorporeal membrane oxygenation (VA-ECMO) as the last therapeutic step after inotropes and invasive mechanical ventilation; in emergency situations, it is a suitable option.1 In respiratory disease, there is increasing use of support with venovenous ECMO implanted in conscious patients who therefore have spontaneous breathing (awake ECMO). This modality aims to avoid intubation, invasive mechanical ventilation, and the associated sedation and immobility, and there is evidence of better postoperative outcomes in terms of complications and mortality.2,3 In the current literature, there is very limited evidence on VA-ECMO in CS. So far, only isolated cases and 3 small series (2 in adults and 1 in children) have shown that it is viable and has good outcomes as a rescue for acute CS (23 patients; 6-month survival, 70.8%)4 and in patients with advanced heart failure and INTERMACS profile 1 who require support as a bridge to device implantation (19 patients; 1-year survival, 84.2%).5
We present the positive experience from our hospital with a group of patients with CS treated with awake VA-ECMO. Of the 73 VA-ECMO implantations for CS between 2010 and 2018, 10 (13.7%) were implanted in patients with spontaneous breathing; 70% of these were in men, with a median [interquartile range] age of 50 [47-57] years. The median APACHE-II score was 16 [9-19], and the SAPS-II score, 30 [25-32]. The most common cause of CS was decompensation of advanced heart failure in patients awaiting transplant (7 patients; 71% in INTERMACS 1 and 29% in INTERMACS 2) and the rest were: 1 acute myocardial infarction in Killip class IV, 1 septic cardiomyopathy, and 1 acute myocarditis (all 3 were in INTERMACS 1). The aim of circulatory support in the first 8 patients was as a bridge to transplant (7) or to decision on definitive treatment (1); 6 (75%) of these patients finally received a transplant and 2 died while on circulatory support (1 due to malignant cerebral infarction and 1 due to refractory multiorgan failure). In the patients with septic myocardiopathy and acute myocarditis, the aim was as a bridge to recovery. Eight patients had implantation of an intra-aortic balloon pump: 5 had already had this implanted prior to ECMO support (those in INTERMACS 1), and 3 required it while on support to offload the left ventricle (table 1).
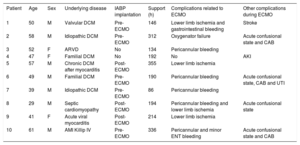
Summary of patients and clinical course
| Patient | Age | Sex | Underlying disease | IABP implantation | Support (h) | Complications related to ECMO | Other complications during ECMO |
|---|---|---|---|---|---|---|---|
| 1 | 50 | M | Valvular DCM | Pre-ECMO | 146 | Lower limb ischemia and gastrointestinal bleeding | Stroke |
| 2 | 58 | M | Idiopathic DCM | Pre-ECMO | 312 | Oxygenator failure | Acute confusional state and CAB |
| 3 | 52 | F | ARVD | No | 134 | Pericannular bleeding | |
| 4 | 47 | F | Familial DCM | No | 192 | No | AKI |
| 5 | 57 | M | Chronic DCM after myocarditis | Post-ECMO | 355 | Lower limb ischemia | |
| 6 | 49 | M | Familial DCM | Pre-ECMO | 190 | Pericannular bleeding | Acute confusional state, CAB and UTI |
| 7 | 39 | M | Idiopathic DCM | Pre-ECMO | 86 | Pericannular bleeding | |
| 8 | 29 | M | Septic cardiomyopathy | Post-ECMO | 194 | Pericannular bleeding and lower limb ischemia | Acute confusional state |
| 9 | 41 | F | Acute viral myocarditis | Post-ECMO | 214 | Lower limb ischemia | |
| 10 | 61 | M | AMI Killip IV | Pre-ECMO | 336 | Pericannular and minor ENT bleeding | Acute confusional state and CAB |
AKI, acute kidney injury; AMI, acute myocardial infarction; ARVD, arrhythmogenic right ventricular dysplasia; CAB, intravascular catheter-associated bacteremia; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenator; ENT, ear nose and throat; F, female; IABP, intra-aortic balloon pump; IMV, invasive mechanical ventilation; M, male; UTI, urinary tract infection.
All ECMO implantations were performed in the intensive care unit with a femorofemoral configuration, except 1 which was implanted in the operating room with a femoroaxillary configuration. The median duration of support was 8 [6.5-11.9] days. Weaning was possible in 80% of the patients (all except the 2 who died), and the overall survival in the intensive care unit was 60%, while survival to discharge from hospital was 50%, similar to that of our general VA-ECMO series.6 While on ECMO, 3 patients required intubation and invasive mechanical ventilation due to acute pulmonary edema (2) or stroke (1) (table 2), with a median duration of invasive mechanical ventilation of 84 [57-96] hours; in our general series, this was 312 [126-564] hours. Prior to their intubation, these patients had been on awake ECMO for 6, 60, and 64hours. The median stay in ICU was 16 [14-18] days and the median hospital stay was 37 [28-59] days.
Summary of ventricular support used
| Cause of cardiogenic shock | Aim of ECMO support | Reason for stopping ECMO | Final outcome of patients weaned from ECMO | Indication for subsequent IMV |
|---|---|---|---|---|
| Decompensation of advanced CHF (7):5 INTERMACS 12 INTERMACS 2 | Bridge to transplant (6)Bridge to decision (1) | Transplant (5)Death (2):1 malignant cerebral infarction1 refractory MOF | Alive (3)Died (2):1 invasive aspergillosis1 postoperative mediastinitis | Surgical intervention* (5)Neurological decline (1)APE (1) |
| AMI Killip IV INTERMACS 1 (1) | Bridge to transplant | Transplant | Death (postoperative aortic rupture) | Surgical intervention* |
| Septic cardiomyopathy INTERMACS 1 (1)Acute myocarditis INTERMACS 1 (1) | Bridge to recovery (2) | Recovery (2) | Alive (2) | APEIMV not required |
AMI, acute myocardial infarction; APE, acute pulmonary edema; CHF, chronic heart failure; ECMO, extracorporeal membrane oxygenation; IMV, invasive mechanical ventilation; MOF, multiorgan failure.
Regarding complications, 9 patients (90%) had at least 1 complication associated with their circulatory support or clinical condition, with a distribution similar to that published in the literature. The most common complication was pericannular bleeding (5), followed by lower limb ischemia (4), other nonintracranial hemorrhage (n), stroke (1), and oxygenator failure (1). Other complications included acute kidney injury in 60% (5 before starting support and 1 after, who required renal replacement therapy, with complete recovery in 100%), infections in 40% (3 associated with the intravascular catheter and 1 urinary), acute confusional state in 4, and stroke (table 1). However, it should be noted here that there were no incidences of respiratory infection or weakness in the critically ill patient (in our general series on VA-ECMO, the incidences were 23% and 20%, respectively, similar to the current literature). Mortality in the patients weaned from ECMO after cardiac transplant related to postoperative mortality.
We present the first published series of awake VA-ECMO in patients with CS in Spain, and the results are promising. In conclusion, and taking into account the limitations of a case series, our results are consistent with the limited international literature. This series demonstrates that VA-ECMO implantation—at least in hospitals with sufficient experience in the management of extracorporeal support in awake patients with spontaneous breathing as circulatory support in CS—is a viable therapeutic option with good outcomes: fewer complications associated with sedation and invasive mechanical ventilation and good morbidity and mortality outcomes. However, more studies are needed to reinforce this practice and optimize appropriate patient selection.