We have previously demonstrated that combination therapy with remote ischemic conditioning (RIC) and metabolic treatment (glucose-insulin-potassium [GIK] or exenatide [a mimetic of the incretin glucagon-like peptide-1]) result in additive protection against infarction in in situ pig hearts, due to different impacts on key cardioprotective pathways.1 However, any proarrhythmic effect of this treatment would limit its clinical applicability. In this work, we analyzed the electrocardiographic recordings obtained in our previous study,1 conducted in an in situ pig model of transient coronary occlusion, to assess the effects of combining RIC with either GIK or exenatide on ventricular ischemia-reperfusion arrhythmias. The effects of these combinations on ventricular arrhythmogenesis were unknown.
In brief, 46 hybrid farm pigs (25-30 Kg) were anaesthetized with intravenous propofol-lipuro 1% and fentanyl and submitted to 40minutes of left anterior descending coronary artery occlusion followed by 2hours of reperfusion (n=7-10/group), as previously described.1 The animals were included in the following groups1: control, RIC, glucose-insulin-potassium (GIK), exenatide, RIC+GIK or exenatide+RIC. Electrocardiographic and hemodynamic recordings were analyzed to determine the incidence of ventricular tachycardia (VT) (4 or more consecutive premature beats of ventricular origin, heart rate faster than 100 beats/min, wide QRS durations [> 120ms]), sustained VT (lasting more than 30 s) and ventricular fibrillation. Connexin 43 expression was assessed in cardiac extracts obtained 5minutes after reperfusion from additional animals (n=4/group).1 Results are expressed as mean±SEM. Arrhythmias and Western blot data were analyzed by nonparametric Krustal Wallis tests and hierarchical ANOVA, and stepwise regression analysis and 1-way ANOVA, respectively.
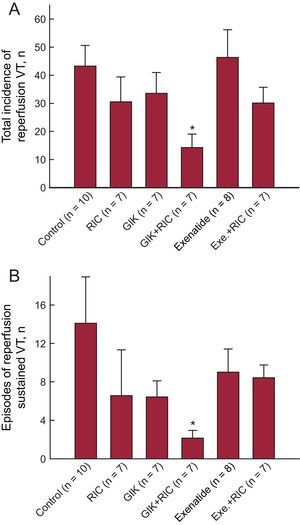
Contrary to previous reports,2,3 in our porcine model none of the 3 individual treatments tested was able to modify the incidence of arrhythmias during myocardial ischemia-reperfusion. However, combined treatment with GIK and RIC significantly reduced the total incidence of VT during reperfusion (Figure 1A) and the number of sustained VT during this phase (Figure 1B). Factorial analysis demonstrated that combination therapy (2 treatments) was the most effective treatment in reducing the number of VT during reperfusion (43.30±7.30, 37.27±4.87, and 22.21±4.08 for none, 1 and 2 treatments, P<.05). However, an important limitation of this work is the use of a single electrocardiogram lead to diagnose VT. This might have induced confusion with other entities such as atrial tachycardia or rapid idioventricular rhythms. To minimize such bias, we took care to include, in the VT definition, criteria such as heart rate and QRS width. On the other hand, no changes were observed with any of the tested treatments on the incidence of VT during the ischemic phase or on the incidence of ventricular fibrillation either during ischemia or reperfusion.
Total number of ventricular (A) and sustained VT (> 30s) (B) during reperfusion in pigs from the 6 experimental groups. Exe, exenatide; GIK, glucose-insulin-potassium; RIC, remote ischemic conditioning; VT, ventricular tachycardia. *(P < .05) indicates significant differences vs control animals.
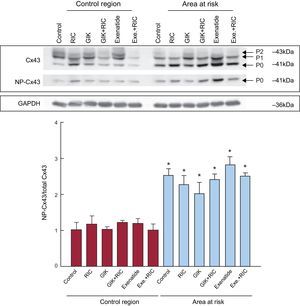
Myocardial connexin 43 and its phosphorylation play a key role in arrhythmogenesis.4 An increase in connexin 43 levels or in its phosphorylation would induce an improvement in gap junctional communication and action potential propagation, and thus might explain the reduction in reperfusion VT seen with combined therapy. However, ischemia-reperfusion associated with connexin 43 dephosphorylation in the area at risk 5minutes after reperfusion (Figure 2) was not modified by any treatment. In contrast, previous studies demonstrated that RIC is associated with increased connexin 43 phosphorylation 2hours after reperfusion in the area at risk in rat hearts.5 Our data thus exclude a role for improved connexin 43-mediated communication in the observed protection against ventricular arrhythmias.
Total and NP connexin 43 (C x 43) expression in control regions and in the area at risk, after 5minutes of reperfusion. Cx43, connexin 43; Exe, exenatide; GIK, glucose-insulin-potassium; NP, nonphosphorylated; RIC, remote ischemic conditioning. *(P<.05) indicates a significant increase in the ratio of nonphosphorylated to total connexin 43 compared with the control region.
This study demonstrates that a combination of RIC with metabolic treatments lacks any proarrhythmic effect. In fact, combination therapy (especially RIC+GIK), has a protective effect against reperfusion arrhythmias, independently of its actions on connexin 43 phosphorylation. In this regard, although a number of experimental cardioprotective therapies have been tested in the clinical setting in patients with acute myocardial infarction,6 few have been demonstrated to improve clinical outcomes. From the present study it is difficult to guarantee the effectiveness of these treatments in patients, but combination therapy is expected to increase the likelihood of success.
FUNDINGThis study was supported by the Spanish Ministry of Science (RETICS-RIC, RD12/0042/0021) and Instituto de Salud Carlos III (PI14/01431).
CONFLICTS OF INTERESTA. Rodríguez-Sinovas has a Miguel Servet contract and I. Barba is a Ramón y Cajal Fellow.