To the Editor,
Vernakalant is a novel antiarrhythmic drug that has proved its efficacy at restoring sinus rhythm in recent-onset atrial fibrillation (AF). Its mechanism of action is based on selective partial blocking of potassium currents in the atrial myocardium, prolonging the atrial refractory period without significantly affecting ventricular refractoriness. This makes it potentially beneficial as a low-effect ventricular proarrhythmogenic, even in patients with structural heart disease.
Class IC drugs–used to restore sinus rhythm and prevent AF recurrence–favor the appearance of flutter, often with 1:1 ventricular conduction. Vernakalant also appears to favor the development of flutter, although 1:1 atrioventricular conduction has not been reported to date.
We present the case of a 77-year-old man with coronary disease without infarction, with hypertensive cardiopathy, normal ventricular function, and 1 previous episode of persistent AF requiring electrical cardioversion. Treatment was with acenocoumarol, enalapril, and simvastatin.
The patient was referred for electrophysiologic study for typical paroxystic atrial flutter with 240ms cycle length in the electrocardiogram.
Initially in sinus rhythm, during placement of the circular multipolar catheter used to record electrocardiograms and for stimulation he developed sustained AF after a 10-min observation period.
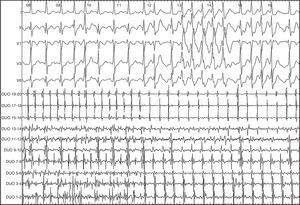
We decided to use intravenous vernakalant for cardioversion. After a first 3mg/kg infusion, the AF organized into flutter with 320ms cycle length, descending atrial activation sequence in the anterior right atrium, and exact return cycle in the cavotricuspid isthmus, compatible with typical flutter (Figure 1).
Figure 1. Surface and intracavity electrocardiogram of 12-pole catheter showing the change from atrial fibrillation to flutter.
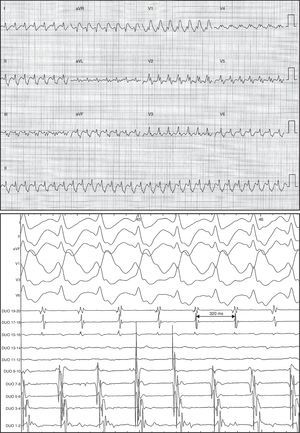
Atrioventricular conduction was initially variable, later stabilizing to 1:1 with right bundle branch block (Figure 2).
Figure 2. Electrocardiogram and intracavity images of flutter with descending atrial activation sequence in anterior surface, 320ms cycle length, 1:1 atrioventricular conduction, and right bundle branch block.
Given good tolerance despite rapid ventricular frequency, we decided to interrupt the drug infusion and perform radiofrequency ablation of the cavotricuspid isthmus.
The Class IC antiarrhythmic drugs flecainide and propafenone slow atrial conduction by blocking voltage-dependent rapid sodium channels, favoring the stability of macro re-entry circuits in anatomic regions with predisposed structures (IC flutter).1
In the right atrium, they condition the slowing of atrial conduction and limit transversal conduction through the crista terminalis, facilitating the appearance of flutter circuits around the tricuspid annulus.
Due to atrial conduction slowing, IC flutter is usually slow and can be led 1:1 to the ventricles. This greatly accelerates ventricular frequency that is often accompanied by aberrant conduction, which can condition poor hemodynamic tolerance of the arrhythmia.
Although experience in the clinical use of vernakalant is very limited, data on its efficacy and safety in 4 controlled clinical trials has been published.2, 3, 4
Vernakalant has demonstrated efficacy superior to a placebo plus amiodarone in cardioversion of recent-onset AF (52% vs 4%-5% at 90-min observation) with 8% post-dose incidence of atrial flutter–which is far superior to amiodarone (0.9%), above all in patients receiving antiarrhythmic drugs.4
Flutter usually appears in the transition to sinus rhythm. To date, neither poor tolerance nor 1:1 atrioventricular conduction have been reported; hence, suspending drug administration when flutter appears seems unnecessary.
One recent study did not demonstrate the efficacy of vernakalant in the cardioversion of flutter.5 The present study has identified a significantly prolonged flutter cycle length (mean, 55ms), accompanied by the slight slowing of atrioventricular conduction, which explains why–at the time of writing–1:1 atrioventricular conduction has not been reported.
The principle mechanism of action of vernakalant is based on blocking potassium currents present in the atrium (IKur and IK,ACh), prolonging the refractory period and potential for atrial action, which produces antifibrillatory action.6 Moreover, in vitro studies have proven vernakalant blocks voltage-dependent sodium channels and that inhibition is greater at higher heart rates–as in AF.
In this patient, slowing atrial conduction–possibly due to sodium channel blocking–could have favored the appearance of slow flutter, which permitted 1:1 conduction to the ventricles.
Although infrequent, this pattern of proarrhythmia should be borne in mind given the foreseeable expansion in the use of vernakalant even though, in our opinion, it does not limit the drug's clinical usefulness.
Corresponding author: martaderiva@gmail.com