Currently, the clinical course of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains uncertain, particularly given the variety of chronic symptoms in the subsequent weeks and months.1 Parameters such as ventilatory efficiency and exercise capacity allow objective assessment of an individual's ventilatory and functional response, and also provide prognostic information on their clinical status, with important implications for treatment.2
The aim of the present study was to examine the—as yet unassessed—effect of persistent coronavirus disease 19 (COVID-19) on parameters of ventilatory efficiency and exercise capacity, in comparison with a group of patients with no history of COVID-19. The sample for this exploratory observational study included 95 individuals (77% were women) with a diagnosis of COVID-19 and mild or moderate symptoms, who had not previously been hospitalized, and had no structural heart disease or lung disease. Patients were considered to have persistent COVID-19 on the basis of compatible signs or symptoms and a positive polymerase chain reaction test for SARS-CoV-2. In addition, they were required to have symptoms persisting for 3 months after the infection, as assessed with a semistructured questionnaire previously used and validated by international expert consensus, which included self-diagnosis of 21 relevant symptoms 3 months after infection (yes/no answers).3
The group of patients with no history of COVID-19 (n=95; 54% women) had not had SARS-CoV-2 infection and were recruited from the exercise capacity and cardiometabolic risk assessment clinic in our hospital. They underwent clinical assessment and functional testing of resting calorimetry, ergospirometry, vascular function, and body composition. Patients were also asked about their physical activity level. The study was approved by the ethics committee of Hospital Universitario de Navarra, and the participants gave signed informed consent (PI_2020/140).
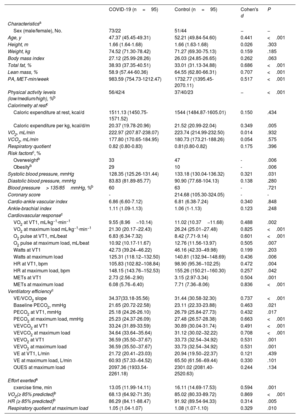
The most prevalent persistent symptom was chronic fatigue (96.1%), followed by headache (81.4%), memory loss (80.4%), and difficulty concentrating (79.4%), the same symptoms as observed in previous studies.4,5 The results of the univariate general linear model (ANCOVA), adjusted for age, sex, and body mass index, showed that, during exercise, the group with persistent COVID-19 had lower oxygen uptake and metabolic equivalents (METs), as well as significantly higher oxygen pulse, the ratio between oxygen uptake and heart rate (VO2/HR), at the first ventilatory threshold (VT1) and at maximum load (P < .01). Significant between-group differences were also observed at peak VO2, as well as in the pulmonary ventilation (VE)/CO2 output (VCO2) slope (d=0.708), the VE/VO2 slope (d=0.531), watts (d=0.436), VE (d=0.257), VO2/HR (d=0.424), METs (d=0.836), and heart rate (HR) as percentage of predicted (d=0.314) (table 1). Approximately 85% of the patients with COVID-19 had a moderate/severe ventilatory limitation score (table 2).
Clinical characteristics and ergospirometry parameters of the study population by group
| COVID-19 (n=95) | Control (n=95) | Cohen's d | P | |
|---|---|---|---|---|
| Characteristicsa | ||||
| Sex (male/female), No. | 73/22 | 51/44 | − | − |
| Age, y | 47.37 (45.45-49.31) | 52.21 (49.84-54.60) | 0.441 | <.001 |
| Height, m | 1.66 (1.64-1.68) | 1.66 (1.63-1.68) | 0.026 | .303 |
| Weight, kg | 74.52 (71.30-78.42) | 71.27 (69.30-75.13) | 0.159 | .185 |
| Body mass index | 27.12 (25.99-28.26) | 26.03 (24.85-26.65) | 0.262 | .063 |
| Total fat, % | 38.93 (37.35-40.51) | 33.01 (31.13-34.88) | 0.686 | <.001 |
| Lean mass, % | 58.9 (57.44-60.36) | 64.55 (62.80-66.31) | 0.707 | <.001 |
| PA, MET-min/week | 983.59 (754.73-1212.47) | 1732.77 (1395.45-2070.11) | 0.517 | <.001 |
| Physical activity levels (low/medium/high), %b | 56/42/4 | 37/40/23 | − | <.001 |
| Calorimetry at restc | ||||
| Caloric expenditure at rest, kcal/d | 1511.13 (1450.75-1571.52) | 1544 (1484.87-1605.01) | 0.150 | .434 |
| Caloric expenditure per kg, kcal/d/m | 20.37 (19.78-20.96) | 21.52 (20.99-22.04) | 0.349 | .005 |
| VO2, mL/min | 222.97 (207.87-238.07) | 223.74 (214.99-232.50) | 0.014 | .932 |
| VCO2, mL/min | 177.80 (170.65-184.95) | 180.73 (173.21-188.26) | 0.054 | .575 |
| Respiratory quotient | 0.82 (0.80-0.83) | 0.81(0.80-0.82) | 0.175 | .396 |
| Risk factorsc, % | ||||
| Overweightb | 33 | 47 | - | .006 |
| Obesityb | 29 | 10 | - | .006 |
| Systolic blood pressure, mmHg | 128.35 (125.26-131.44) | 133.18 (130.04-136.32) | 0.321 | .031 |
| Diastolic blood pressure, mmHg | 83.83 (81.89-85.77) | 90.90 (77.68-104.13) | 0.138 | .280 |
| Blood pressure> 135/85mmHg, %b | 60 | 63 | - | .721 |
| Coronary score | - | 214.68 (105.30-324.05) | - | - |
| Cardio-ankle vascular index | 6.86 (6.60-7.12) | 6.81 (6.38-7.24) | 0.340 | .848 |
| Ankle-brachial index | 1.11 (1.09-1.13) | 1.06 (1-1.13) | 0.123 | .248 |
| Cardiovascular responsec | ||||
| VO2 at VT1, mL/kg−1·min−1 | 9.55 (8.96−10.14) | 11.02 (10.37−11.68) | 0.488 | .002 |
| VO2 at maximum load mL/kg−1·min−1 | 21.30 (20.17−22.43) | 26.24 (25.01−27.48) | 0.825 | <.001 |
| O2 pulse at VT1, mL/beat | 6.83 (6.34-7.32) | 8.42 (7.71-9.14) | 0.601 | <.001 |
| O2 pulse at maximum load, mL/beat | 10.92 (10.17-11.67) | 12.76 (11.56-13.97) | 0.505 | .007 |
| Watts at VT1 | 42.73 (39.24−46.22) | 46.16 (42.33−49.98) | 0.199 | .203 |
| Watts at maximum load | 125.31 (118.12−132.50) | 140.81 (132.94−148.69) | 0.436 | .006 |
| HR at VT1, bpm | 105.83 (102.82−108.84) | 98.90 (95.36−102.25) | 0.472 | .004 |
| HR at maximum load, bpm | 148.15 (143.76−152.53) | 155.26 (150.21−160.30) | 0.257 | .042 |
| METs at VT1 | 2.73 (2.56−2.90) | 3.15 (2.97-3.34) | 0.504 | .001 |
| METs at maximum load | 6.08 (5.76−6.40) | 7.71 (7.36−8.06) | 0.836 | <.001 |
| Ventilatory efficiencyc | ||||
| VE/VCO2 slope | 34.37(33.18-35.56) | 31.44 (30.58-32.30) | 0.737 | <.001 |
| Baseline PECO2, mmHg | 21.65 (20.72-22.58) | 23.11 (22.33-23.88) | 0.463 | .021 |
| PECO2 at VT1, mmHg | 25.18 (24.26-26.10) | 26.79 (25.84-27.73) | 0.432 | .017 |
| PECO2 at maximum load, mmHg | 25.23 (24.37-26.09) | 27.48 (26.57-28.38) | 0.663 | <.001 |
| VEVCO2 at VT1 | 33.24 (31.89-33.59) | 30.89 (30.04-31.74) | 0.491 | <.001 |
| VEVCO2 at maximum load | 34.64 (33.64−35.64) | 31.12 (30.02−32.22) | 0.708 | <.001 |
| VEVO2 at VT1 | 36.59 (35.50−37.67) | 33.73 (32.54−34.92) | 0.531 | .001 |
| VEVO2 at maximum load | 36.59 (35.50−37.67) | 33.73 (32.54−34.92) | 0.531 | .001 |
| VE at VT1, L/min | 21.72 (20.41−23.03) | 20.94 (19.50−22.37) | 0.121 | .439 |
| VE at maximum load, L/min | 60.93 (57.33−64.52) | 65.50 (61.56−69.44) | 0.330 | .101 |
| OUES at maximum load | 2097.36 (1933.54-2261.18) | 2301.02 (2081.40-2520.63) | 0.244 | .134 |
| Effort exerteda | ||||
| Exercise time, min | 13.05 (11.99-14.11) | 16.11 (14.69-17.53) | 0.594 | .001 |
| VO2(≥ 85% predicted)b | 68.13 (64.92-71.35) | 85.02 (80.33-89.72) | 0.869 | <.001 |
| HR (≥ 85% predicted)b | 86.29 (84.11-88.47) | 91.92 (89.54-94.33) | 0.314 | .005 |
| Respiratory quotient at maximum load | 1.05 (1.04-1.07) | 1.08 (1.07-1.10) | 0.329 | .010 |
HR, heart rate; METs, metabolic equivalents; OUES, oxygen uptake efficiency slope; PA, physical activity; PECO2, expired CO2 pressure; VE/VCO2, slope of the pulmonary ventilation and VCO2 ratio; VEVCO2, ventilatory equivalent for CO2, VEVO2, ventilatory equivalent for O2; VO2, oxygen uptake; VT1, first ventilatory threshold.
Data are presented as mean and 95% confidence intervals (95% CI) without adjustment or percentage as appropriate.
Data presented as marginal mean and 95% CI. General linear univariate model (ANCOVA), adjusted for age, sex, and body mass index. The ergospirometry test on cycle ergometer (Lode Excalibur Sport, Germany) consisted of incremental ramp increases in load, starting with 25 W with 25-W increments every 2min (pedaling cadence, 50-60 revolutions/min). The variables VO2 (mL/kg−1·min−1), oxygen pulse (VO2/HR), parameters VE and VT (L/min−1), ventilatory equivalents of O2 and CO2 (VEVO2, VEVCO2), and expiratory CO2 pressure (PECO2) were recorded at the first ventilatory threshold (VT1) and at maximum load using flow analysis and concentrations of inhaled and exhaled respiratory gases in the mixing chamber (QUARK CPET, Cosmed, Italy).
Comparison of ergospirometry criteria and ventilatory performance score by study group
| Criteria | Categories | COVID-19 (n=95)* | Control (n=95)* | χ2 | P | ||
|---|---|---|---|---|---|---|---|
| VO2 inflection at VT1a | Normal > 11, mL/kg/min | 29 | (30) | 44 | (46) | 4.587 | .006 |
| Abnormal < 11, mL/kg/min | 67 | (70) | 51 | (54) | |||
| VE/VCO2b | Normal < 34, slope in degrees | 51 | (54) | 74 | (77) | 11.318 | .001 |
| Abnormal > 34, slope in degrees | 44 | (46) | 21 | (23) | |||
| OUESc | Normal > 1550 mL | 65 | (68) | 72 | (76) | 0.942 | .331 |
| Abnormal < 1550 mL | 30 | (32) | 23 | (24) | |||
| COPd | Normal < 30 L | 85 | (89) | 95 | (100) | 8.550 | .003 |
| Abnormal > 30 L | 10 | (11) | 0 | 0 | |||
| ΔVO2/HR VT2 vs VT1e | Normal > 0 | 92 | (97) | 89 | (94) | 0.467 | .494 |
| Abnormal < 0 | 3 | (3) | 6 | (6) | |||
| Ventilatory performance scoref | No limitation | 14 | (15) | 29 | (31) | 9.847 | .007 |
| Moderate limitation | 62 | (65) | 58 | (61) | |||
| Severe limitation | 19 | (20) | 8 | (8) | |||
COP, cardiorespiratory optimal point; HR, heart rate; OUES, oxygen uptake efficiency slope; VCO2, carbon dioxide produced; VE, pulmonary ventilation; VO2, oxygen uptake; VT1, first ventilatory threshold; VT2, second ventilatory threshold.
Point of inflection of VO2 expressed in mL/kg/min and estimated manually on the graph of VO2 at VT1.
Ventilatory performance criteria score was derived from the sum of the abnormal criteria in a-e, then classified as: no ventilatory limitation (no abnormal criteria), moderate limitation (1-2 abnormal criteria), and severe limitation (more than 3 abnormal criteria).
Values are expressed as No. (%).
In previous studies,1 patients with COVID-19 showed peak VO2 values that were 35% lower (∼15mL/kg-1·min-1) than the control group (∼23mL/kg-1·min-1) at 30 days after hospital discharge. Debeaumont et al.4 reported on parameters of VO2 and maximum power of, respectively, ∼80% and ∼90% of predicted values for age at 6 months after discharge. Similarly, patients with persistent COVID-19 symptoms had a significant reduction in 6-minute walk test at 6 months after onset of symptoms.5 In our series, the COVID-19 group showed peak VO2 values ∼18% lower than the control group. There was also a mixed pattern of abnormalities in parameters of ventilatory efficiency including VO2 at VT1 (70% vs 54%), abnormal VE/VCO2 (46% vs 36%), and a very low VE/VCO2 ratio (COP) (11% vs 0%), indicating a higher risk of functional deterioration.
To date, the mechanisms to explain the reduced exercise capacity in patients with persistent COVID-19 are unknown, but it has been hypothesized that excess adiposity (as seen in this series) and low levels of physical activity could partly explain the findings of this study.1 The myopathic effect of SARS-CoV-2 has also not been excluded as a cause of functional deterioration in patients after COVID-19.2 However, experimental studies are needed to corroborate these hypotheses.2,4 The main limitations of our study are the number of patients included, the inclusion of a majority of women (a characteristic of persistent COVID-19 syndrome) and the lack of previous measures of exercise capacity, a limitation that is difficult to solve given the emergent nature of the pandemic.
More research is needed to better understand the long-term consequences of COVID-19 on functional capacity over the whole spectrum of the disease, especially the underlying biological mechanisms that characterize its pathophysiology. Considering the central role of exercise capacity in patients with persistent COVID-19, exercise rehabilitation could be fundamental in this new and little-known situation. Therefore, it is essential to establish strategies with multicomponent programs, to optimize recovery in these patients.
FUNDINGThis study was funded in part by a grant (PID2020-113098RB-I00) corresponding to the call for RD&I projects from the national programs for knowledge generation and scientific and technical strengthening of the RD&I system aimed at the challenges of society, within the framework of the National Plan for scientific and technical research and innovation 2017-2020.
AUTHORS’ CONTRIBUTIONSAll authors contributed substantially to the concept and design, data acquisition, analysis, and interpretation, as well as the writing, review, and intellectual content of the manuscript.
CONFLICTS OF INTERESTSThe authors have no conflict of interests to declare.