With the recent incorporation of the Micra (Medtronic) leadless transcatheter pacemaker into clinical practice,1 we believe that the role of cardiac imagining techniques in identifying and monitoring possible postimplantation complications is of considerable interest. Echocardiography is the technique of choice for diagnosing complications secondary to implantation of intracardiac devices.2,3 It can establish the position of the electrode tip in the right ventricle (RV), sometimes by following the course of the lead, and determine the relationship between the electrode and the tricuspid valve apparatus.4 Identifying the position of the Micra capsule is an imaging challenge.
The Micra transcatheter pacemaker is a miniaturized, single-chamber pacing system that provides bipolar sensing and pacing to the RV. The device is contained in a capsule whose volume is 0.8 cm, length 25.9 mm, external diameter 6.7 mm, and weight 2.0 g. The Micra has a fixation mechanism composed of 4 electrically inactive nitinol tines, designed to attach to the cardiac tissue at the selected RV site. The potential implantation sites include the septal-apical and midseptal regions and, less commonly, the RV outflow tract. The following are examples of each of these implantation locations, with the corresponding 3-dimensional transthoracic echocardiography (3D-TTE) image.
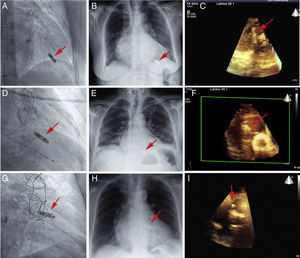
Case 1 is an 82-year-old woman with mitral regurgitation and permanent atrial fibrillation, who experienced recurrent syncope and significant pauses on Holter monitoring. The radiographic and 4-chamber 3D-TTE images show the position of the device in the septal-apical region of the RV (Figure 1A, Figure 1B, Figure 1C and video 1 of the supplementary material).
Right anterior oblique view of the leadless pacemaker during implantation, the follow-up chest radiograph (posterior-anterior view), and the image of the device (arrow) on 3-dimensional transthoracic echocardiography examination in the septal-apical (A-C), midseptal (D-F), and right ventricular outflow tract (G-I) positions. 3D, 3-dimensional.
Case 2 is an 81-year-old man with severe, symptomatic aortic valve stenosis and permanent atrial fibrillation. After percutaneous implantation of a CoreValve aortic prosthesis, he experienced complete atrioventricular block requiring pacing with a temporary intravenous pacemaker. The Micra was placed in the midseptal region (Figure 1C and Figure 1E, arrow). The 3D-TTE image, which depicts the right heart chambers in a longitudinal view (Figure 1F and video 2 of the supplementary material), shows the device and its proximity to the tricuspid valve apparatus.
Case 3 is a 74-year-old woman with surgically revascularized ischemic heart disease, who had refractory angina precipitated by episodes of left atrial tachycardia, with a rapid ventricular response. The device was placed in the RV outflow tract (due to elevated capture thresholds on initial placement in a midseptal position). Following withdrawal of the deployment mechanism, atrioventricular node ablation was carried out in the same procedure through the system's long introducer. The radiographic images and 3D-TTE in a high, longitudinal parasternal view at the level of the great vessels shows the location of the device in the RV outflow tract (Figure 1G, Figure 1H and video 3 of the supplementary material).
Leadless pacemaker implantation is feasible and safe, and provides potential advantages over conventional systems, although studies with a longer follow-up will be needed before the use of these devices becomes widespread in daily clinical practice.1 The main indication is for patients who exclusively require ventricular pacing and have a special risk related to implantation of conventional pacemakers, in particular those receiving oral anticoagulant agents and those with prosthetic valves, as the risk of producing hematomas is minimized with the leadless pacemaker.5,6 Although 3D-TTE has poorer spatial resolution than other imaging techniques, the Micra capsule is hyperechoic, which facilitates determination of its location and orientation. It should be specified whether the device is in a septal-apical or midseptal location, or in relation with the RV outflow tract. In conventional pacing systems, the orientation in these locations can be toward the septum or more laterally, toward the free wall.2 In the case of the Micra pacemaker, a septal position should always be sought, avoiding the RV free wall because of the potential risk of cardiac perforation and tamponade (Figure 1).
A systematic analysis by 2-dimensional and 3-dimensional echocardiography should include the 4 parasternal short-axis (transverse) views, the level of the great vessels, mitral valve (implants in the outflow tract), and papillary muscles (midseptal implants), and an apical view, as well as a modified parasternal long-axis (longitudinal) view toward the right chambers (anterior angulation) and a modified apical 4-chamber view, also toward the right chambers (right anterior angulation). If there is a poor acoustic window, a subcostal approach can be used. Compared with 2-dimensional echocardiography, 3D-TTE facilitates localization of the capsule, as it can precisely define the implantation site, determine whether the device is stable, and depict the relationships between the device and other RV structures, the moderator band, trabeculae, and tricuspid valve. This capability can be useful during implantation in difficult cases and during follow-up to monitor possible complications.
We believe that the foreseeable widespread use of Micra systems should be associated with correct characterization of the implantation site. For this purpose, a protocol-based examination specifying the views to acquire, which should include 3D-TTE.