Hypercholesterolemia continues to be an important comorbidity often seen in heart transplant (HT) recipients and is associated with a higher cardiovascular risk and with the appearance of graft vascular disease (GVD).1 Statin therapy in this patient group has been shown to significantly reduce the incidence of acute graft rejection and GVD and to increase survival, benefits attributable not only to lower plasma cholesterol concentrations but also to the immunomodulatory effects of statins.2 Consequently, clinical practice guidelines recommend long-term use in all HT recipients, regardless of low-density lipoprotein cholesterol (LDL-C) levels.3
Proprotein convertase subtilisin/kexin type 9 (PCSK9) selectively binds to LDL-C receptors in the hepatocyte membrane, enhancing receptor breakdown and indirectly raising plasma LDL-C concentrations. The PCSK9 inhibitors (PCSK9i) known as alirocumab and evolocumab are monoclonal antibodies that block the activity of this protein, and they have been shown to significantly reduce LDL-C and the risk of cardiovascular events.4 Furthermore, PCSK9 metabolism does not involve cytochrome P450 or certain hepatic carriers, such as organic anion transporting polypeptide C (OATP-C), which play a key role in the process that breaks down statins and common immunosuppressant drugs, thereby dramatically reducing the likelihood of drug interactions and adverse reactions. Hence, this pharmacological group is an interesting therapeutic alternative for the treatment of hypercholesterolemia in HT recipients, although there is currently little evidence on its safety and efficacy in this population.
We describe a series of 5 HT recipients who began taking PCSK9i due to LDL-C > 100mg/dL despite ezetimibe therapy and, when tolerated, the highest dose of a statin, as recommended in the therapeutic positioning report published by the Ministry of Health, Consumer Affairs and Social Welfare of the Government of Spain. All patients were men, the mean age was 58.8±8 years, and the mean post-HT time was 13±5.2 years. In all patients, the immunosuppressant regimen included 3 drugs, and in 3 patients (patients 3, 4, and 5), the presence of GVD had been previously confirmed. Patients 1, 2, and 5 were receiving a combination of rosuvastatin 40mg/d and ezetimibe 10mg/d; patient 3 was receiving only ezetimibe 10mg/d due to a history of liver and muscle toxicity with several statins, and patient 4 was receiving a combination of pitavastatin 4mg/d and ezetimibe 10mg/d following muscular toxicity with other, more potent statins (Table 1).
Baseline characteristics and trend in LDL-C levels in HT recipients receiving treatment with a PCSK9i agent (alirocumab 75mg/14d)
| Patient | Age, y | Time since HT, y | IS regimen | Initial statin | Final statin | Duration of treatment with PCSK9i, mo | Baseline LDL-C, mg/dL | Final LDL-C, mg/dL |
|---|---|---|---|---|---|---|---|---|
| 1 | 68 | 9.1 | TAC+EVL+corticosteroids | Rosuvastatin 40 mg/d | Rosuvastatin 20 mg/d | 15 | 175 | 67 |
| 2 | 48 | 9 | CsA+MMF+corticosteroids | Rosuvastatin 40 mg/d | Rosuvastatin 40 mg/d | 5.6 | 180 | 98 |
| 3 | 58 | 10.5 | TAC+EVL+corticosteroids | No | Pitavastatin 1 mg/d | 21.4 | 129 | 69 |
| 4 | 73 | 13.4 | CsA+EVL+corticosteroids | Pitavastatin 4 mg/d | Pitavastatin 4 mg/d | 23.6 | 146 | 35 |
| 5 | 59 | 22.9 | TAC+EVL+corticosteroids | Rosuvastatin 40 mg/d | Rosuvastatin 40 mg/d | 12.3 | 146 | 38 |
CsA, cyclosporin A; EVL, everolimus; HT, heart transplantation; IS, immunosuppression; LDL-C, low-density lipoprotein cholesterol; MMF, mycophenolate mofetil; PCSK9i: proprotein convertase subtilisin/kexin type 9 inhibitor; TAC, tacrolimus.
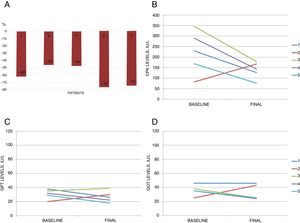
The PCSK9i administered to all patients was subcutaneous alirocumab 75mg/14d, for a mean of 15.6±6.5 months. Mean baseline levels for total cholesterol, LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides were 262±21, 155±19, 60±14, and 227±41mg/dL, respectively, and dropped to 150±33, 61±23, 58±19, and 153±63mg/dL by the end of follow-up. All patients reached the target of LDL-C <100mg/dL, with percent reductions of 45% to 76% (Figure 1).
A, percent reduction in low-density lipoprotein cholesterol after the start of treatment with a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) (alirocumab 75mg/14d). B-D, trend for creatine kinase (CPK), glutamic-pyruvic transaminase (GPT), and glutamic-oxalacetic transaminase (GOT) levels (baseline and end of follow-up) during PCSK9i therapy.
There were no reports of drug-related specific adverse reactions, such as injection site reactions or pruritus, and no abnormalities were observed in the liver panel or in creatine kinase levels. None of the patients had unexplained fluctuations in blood concentrations of immunosuppressant drugs, episodes of graft rejection, or infections. Treatment was discontinued in only 1 patient, at the patient's own request (patient 2). Patient 3 experienced 2 acute non–Q-wave myocardial infarctions at 2 and 7 months after starting PCSK9i and an ischemic stroke at 12 months. The same patient had been hospitalized 3 years earlier due to unstable angina, and coronary angiography revealed GVD affecting 1 vessel, which was revascularized. Additionally, from the time the patient started alirocumab 75mg/14d, he had been taking only ezetimibe 10mg/d due to statin intolerance, with LDL-C levels> 100mg/dL persisting until the third cardiovascular event. At that time, pitavastatin 1mg/d was started; 4 months later, the patient reached the target of LDL-C <100mg/dL, experiencing good tolerance and no new adverse events.
In summary, this is the first series in Spain of HT recipients treated with a PCSK9i. To date, only 2 similar series have been published, one with 6 patients and a mean follow-up of 9 months5 and another with 10 patients and a mean follow-up of 10 months.6 Our series contributes further data with a longer follow-up (nearly 16 months).
These results indicate the potential safety and efficacy of PCSK9i for treating hypercholesterolemia in HT recipients, although larger studies with longer follow-up are needed to confirm this hypothesis and to evaluate the effects in terms of cardiovascular morbidity and mortality.
FUNDINGStudy cofunded by the European Regional Development Fund (ERDF) through the Biomedical Cardiovascular Diseases Network (CIBERCV), Carlos III Health Institute, Madrid, Spain.