We present the first 2 cases of transfemoral transcatheter tricuspid valve replacement performed in Spain.
The patients, both symptomatic, had torrential tricuspid regurgitation (TR), high surgical risk (TRI-SCORE, 34% and 14%), severe dilation, and moderate right ventricular (RV) dysfunction (figure 1A, video 1 of the supplementary material). Surgery was ruled out by the multidisciplinary team and it was decided to attempt a transcatheter approach. Repair was not considered because of the anatomic characteristics (gap size >8mm, marked annular dilatation, significant leaflet restriction, and, in 1 of the patients, rheumatic involvement). The chosen procedure was orthotopic valve replacement with the Cardiovalve system (Cardiovalve, Or-Yehuda, Israel). Both patients consented to the procedure and its publication. Their clinical characteristics are summarized in table 1.
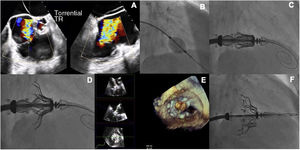
A: midesophageal X-plane transesophageal echocardiographic (TEE) view showing torrential tricuspid regurgitation (TR) over the valve. B: fluoroscopic guidance during insertion of the 12-Fr sheath. C: fluoroscopic guidance during advancement of the introducer and valve along the high-support guidewire towards the valve plane. D: fluoroscopic image showing the open grasping legs in the atrium. E: 3-dimensional TEE multiplanar reconstruction confirming adequate insertion of the native leaflets. F: final fluoroscopic image showing successful deployment of the valve.
Comparison of baseline patient characteristics.
| Baseline characteristic | Patient 1 | Patient 2 |
|---|---|---|
| Age, y | 82 | 71 |
| Sex | Female | Female |
| Previous valve surgery | No | Mechanical mitral and aortic valves |
| Permanent atrial fibrillation | Yes | Yes |
| Anticoagulation | Apixaban | Acenocoumarol |
| Furosemide dose, mg | 60 | 40 |
| Previous TR grade | 5 | 5 |
| Type of TR | Functional | Rheumatic |
| LV systolic function | 55 | 60 |
| RV systolic function | 35 | 39 |
| Tricuspid annulus diameter, mm | 54 × 46 | 52 × 44 |
| Mean PAP, mmHg | 28 | 14 |
| PCP, mmHg | 16 | 10 |
| V wave | 35 | 17 |
| Cardiac output, L/min | 4.4 | 3.0 |
| EuroSCORE II, % | 3.19 | 2.77 |
| TRI-SCORE, % | 34 | 14 |
| NT-proBNP, pg/mL | 1297 | 714 |
| Creatinine, mg/dL | 0.98 | 0.84 |
| ALT, IU/L | 24 | 27 |
| Total bilirubin, mg/dL | 1.02 | 1.06 |
| Hemoglobin, g/dL | 11.6 | 13.8 |
ALT, alanine transaminase; LV, left ventricle; NT-proBNP, amino-terminal fraction of pro-brain natriuretic peptide; PAP, pulmonary artery pressure; PCP, pulmonary capillary pressure; RV, right ventricle; TR, tricuspid regurgitation.
The Cardiovalve system is designed for mitral and tricuspid valve replacement. It consists of a steerable, transfemoral catheter (32-Fr) and a trileaflet bovine pericardial valve sutured using Dacron to a dual (atrial and ventricular) self-expanding, welded nitinol frame for robust radial strength. The structure features 24 grasping legs for atraumatic anchoring of the valve to the native mitral annulus. The valve is available in 3 sizes covering annulus diameters ranging from 36 to 55 mm. Choice of size is based on computed tomography measurements.1
The procedures were performed under general anesthesia with fluoroscopic and transesophageal echocardiographic (TEE) guidance and fusion imaging with HeartNavigator (Philips, The Netherlands).
The right femoral vein was surgically exposed and an 18-Fr catheter (Cook Medical, USA) implanted. Using an Agilis Nxt steerable introducer (Abbott, USA), a pigtail catheter was delivered to the right ventricular apex, enabling advancement of an extra-small, high-support Safari guidewire (Boston Scientific, United States). It is essential that this guidewire does not interfere with the chordae tendineae (figure 1B).
The introducer with the Cardiovalve (extra large in both cases) was then advanced. Using the control wheels, the system was directed towards the valve plane (figure 1C) and raised until the leaflet grasping legs could be opened in the atrium (figure 1D). Once the system was centered, the path was corrected. The system was then advanced into the RV and the native leaflets grasped. Adequate capture was checked using 3-dimensional multiplanar reconstruction (figure 1E, video 2 of the supplementary material). The atrial flange, followed by the ventricular flange, was then released to complete deployment (figure 1F). The results were evaluated by TEE. Successful implantation was achieved in both cases, with no evidence of residual TR and a gradient <3mmHg (videos 3 and 4 of the supplementary material). Recovery was largely uneventful, with 1 patient developing a small hematoma at the vascular access site and the other showing a transient drop in platelet count. They were discharged on days 7 and 10, respectively. Clinical progress was favorable at 3 months in both cases. Follow-up echocardiograms showed correct alignment of the valves, no significant decline in RV function, and an insignificant gradient. Moderate paravalvular regurgitation was observed in 1 of the patients.
TR is a common valve disease with significant clinical consequences (impaired quality of life and increased mortality). Treatments to date have been associated with high morbidity and mortality,2,3 prompting the development of several transcatheter techniques, which have shown promising results. Although repair procedures are currently more common, their limitations impede high success rates.3 The emergence of tricuspid valve replacement has created opportunities for patients who are not candidates for repair procedures, such as annuloplasty or edge-to-edge repair. The initial results observed with transfemoral tricuspid valve replacement are highly encouraging, with high success rates, few complications, and near-complete elimination of TR.4 The Cardiovalve system is a new alternative for tricuspid valve replacement. It has a low profile that does not interfere with the RV structures or outflow tract, comes in 3 sizes compatible with a wide range of annulus diameters, and has multiple leaflet grasping legs to ensure firm anchoring and an atrial sealing flange to minimize the risk of perivalvular leaks.
In conclusion, transcatheter tricuspid valve replacement using the Cardiovalve system may afford an opportunity for treating patients with anatomic limitations impeding other treatments.
FundingNone.
Authors’ ContributionsAll the authors contributed to this work. B. Caneiro-Queija and R. Estévez-Loureiro conceived the paper and collected the data. B. Caneiro-Queija, R. Estévez-Loureiro, and M. Barreiro-Pérez wrote the manuscript and M. Barreiro-Pérez, M. Piñón-Esteban, J. Baz-Alonso, and A. Íñiguez-Romo critically reviewed it. All the authors approved the final version.
Conflicts of InterestNone.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.08.011