An 88-year-old woman with diabetes, hypertension, chronic kidney failure (Cockcroft-Gault 42mL/min), a logistic EuroSCORE of 8% and 2 of the Fried frailty criteria (reversible frailty) was considered unsuitable for surgery by her healthcare center and was referred for transcatheter implantation of an aortic prosthesis1 for severe symptomatic aortic stenosis.2,3 Coronary angiography revealed the absence of lesions and computed tomography showed an iliofemoral axis suitable for transfemoral prosthesis implantation with maximum and minimum aortic annulus diameters of 23.9mm and 16.5mm. Transesophageal echocardiography showed substantial degenerative aortic stenosis, with transaortic gradient>80mmHg, a moderately calcified valve, symmetrical opening and 17-mm annulus.
Vascular access was via the right femoral artery with a 16 Fr delivery catheter. Pre-implantation angiography showed mild-moderate aortic regurgitation and good calcium alignment (Fig. 1). Valve placement was guided by transesophageal echocardiography. A number 23 Edwards-SAPIEN XT prosthesis was advanced, without major difficulty, through the non-predilatated valvular orifice and implantation was performed with 200 bpm overstimulation. Perioperative echocardiography showed optimal prosthesis deployment, without regurgitation, and good placement (Fig. 2). The patient progressed satisfactorily. Follow-up echocardiography showed normal prosthetic function with a maximum gradient of 16mmHg, without regurgitation.
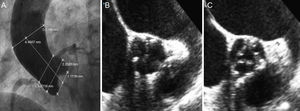
Images prior to percutaneous aortic valve implantation. A: aortogram. B and C: short-axis transesophageal echocardiography at the level of the aortic valve plane that allows visualization of the aortic valve, which is circular with moderate calcification and symmetrical distribution in systole and diastole.
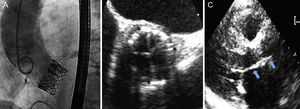
Images following implantation. A: aortogram, with adequate deployment and without aortic regurgitation. B: short-axis perioperative transesophageal echocardiography at the level of the aortic valve plane, with adequate prosthesis expansion (asterisks indicate the valve outline). C: long-axis parasternal follow-up transthoracic echocardiography, with adequate prosthesis implantation (arrows indicate the site of valve implantation).
With a EuroSCORE of 8% and reversible frailty, this patient was considered unsuitable for conventional surgery but suitable for transcatheter implantation,4 even though the transesophageal echocardiography-measured annulus diameter was 17mm (Edwards Lifesciences recommend ≥18mm annulus diameter for Edwards-SAPIEN XT valve implantation). Prior experience with a substantial number of transfemoral prosthesis implantation procedures has shown us that annulus diameter is simply one more factor to be considered when determining patient suitability for transcatheter implantation. Variables such as calcium quantity and distribution, the width of the sinuses of Valsalva, outflow tract diameter or computed tomography-measured annulus diameter (maximum annulus diameter was 23.9mm) must also be analyzed.
Since percutaneous implantation techniques began to be used for aortic valves, previous valvuloplasty has been considered obligatory to facilitate the progress and optimal expansion of the prosthesis.5 Although several cases of direct valve-in-valve implantation of Edwards-SAPIEN prostheses without previous valvuloplasty have been described, we are not aware of any cases of direct native valve implantation in patients with aortic stenosis. Grube et al.6 recently described CoreValve® self-expanding prosthesis (Medtronic; Minneapolis, Minnesota, United States) implantation without valvuloplasty, and concluded that the technique was safe and feasible. Balloon valvuloplasty can cause severe aortic regurgitation and hemodynamic instability in some patients and could favor the migration of calcium particles to the cerebral circulation. Direct implantation without valvuloplasty would avoid these complications in some patients with favorable anatomy (low levels of calcification, homogeneous distribution and symmetrical native valve opening).6 Moreover, given native valve characteristics, prosthesis placement and expansion might be the same or better than when using the conventional technique. In our patient these anatomic criteria were favorable, with moderate valve calcification.
The difficulties that might be encountered during implantation and their possible solutions are as follows:
- •
Advancing the prosthesis through the native valve may not be feasible. Should this occur, it is essential to avoid forcing progress and perform valvuloplasty via the contralateral femoral artery delivery catheter.
- •
Correctly positioning the prosthesis may be difficult if valve calcification is minimal. Hence, we consider the use of echocardiography during implantation to be essential.
- •
Given that the Edwards-SAPIEN XT prosthesis expands by volume, expansion may be insufficient. We believe that slow, progressive balloon inflation, maintained for ≥5s during implantation ensures adequate expansion.
One further advantage—although it was not considered essential when choosing the technique described—is that if this technique is shown to be appropriate for specific patients, kits could be prepared for this patient group, which would cut the cost of packs with no balloon.
This is the first case of transfemoral implantation of an Edwards-SAPIEN XT aortic prosthesis without previous valvuloplasty. We believe it is technically possible and could be safer than traditional implantation in suitable patients (those with low levels of valve calcification, homogeneous calcium distribution and a symmetrical opening). In these patients, some of the complications inherent to valvuloplasty can be avoided. If this initial experience leads to equal levels of safety and efficacy in future patients, eliminating the balloon from the kits prepared for these patients could help lower the costs of transfemoral implantation of this prosthesis.
.