The treatment of patent ductus arteriosus (PDA) in preterm infants continues to be controversial.1,2 Until recently, the only alternative after the failure of medical treatment was surgical ligation. However, because thoracotomy is an independent risk factor for functional disability and/or motor impairment in preterm infants, there is a need for alternative strategies. Recent publications indicate that percutaneous occlusion is feasible in low-weight preterm infants,3–5 but there are limited data on its safety.
The aim of our work was to characterize PDA occlusion with the Amplatzer Duct Occluder II Additional Size (ADO-II-AS) and the incidence of adverse events (both during and after the procedure) using a descriptive study of a preterm infant cohort from January 2011 to December 2016.
A total of 27 patients were included, with a median age of 31 (range, 17-87) days, a median corrected gestational age of 31 (range, 29-37) weeks of life, and a median weight of 1260 (range, 1000-1980) g, with 75% weighing less than 1.5kg.
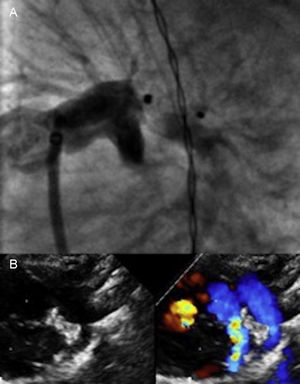
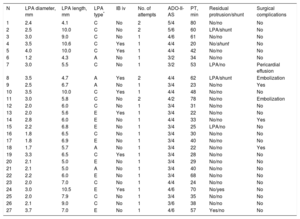
A venous anterograde approach was used in all patients. Procedural data are shown in Table 1. The device was successfully implanted in all patients and there were no major intraprocedural complications. Complete occlusion of the PDA was immediately achieved in 23 patients; 4 patients had a small residual shunt on angiography and echocardiography. Four patients (15%) had slight device protrusion into the left pulmonary artery (LPA) after its release (Figure 1).
Angiographic and Procedural Data
| N | LPA diameter, mm | LPA length, mm | LPA type* | IB iv | No. of attempts | ADO-II-AS | PT, min | Residual protrusion/shunt | Surgical complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.4 | 4.1 | C | No | 2 | 5/4 | 80 | No/no | No |
| 2 | 2.5 | 10.0 | C | No | 2 | 5/6 | 60 | LPA/shunt | No |
| 3 | 3.0 | 9.0 | C | No | 1 | 4/6 | 61 | No/no | No |
| 4 | 3.5 | 10.6 | C | Yes | 1 | 4/4 | 20 | No/shunt | No |
| 5 | 4.0 | 10.0 | C | Yes | 1 | 4/4 | 42 | No/no | No |
| 6 | 1.2 | 4.3 | A | No | 1 | 3/2 | 34 | No/no | No |
| 7 | 3.0 | 5.5 | C | No | 1 | 3/2 | 53 | LPA/no | Pericardial effusion |
| 8 | 3.5 | 4.7 | A | Yes | 2 | 4/4 | 62 | LPA/shunt | Embolization |
| 9 | 2.5 | 6.7 | A | No | 1 | 3/4 | 23 | No/no | Yes |
| 10 | 3.5 | 10.0 | C | Yes | 1 | 4/4 | 48 | No/no | No |
| 11 | 3.0 | 5.8 | C | No | 2 | 4/2 | 78 | No/no | Embolization |
| 12 | 2.0 | 6.0 | C | No | 1 | 3/4 | 31 | No/no | No |
| 13 | 2.0 | 5.6 | E | Yes | 1 | 3/4 | 22 | No/no | No |
| 14 | 2.8 | 6.0 | E | No | 1 | 4/4 | 33 | No/no | Yes |
| 15 | 2.2 | 6.8 | E | No | 1 | 3/4 | 25 | LPA/no | No |
| 16 | 1.8 | 6.5 | C | No | 1 | 3/4 | 30 | No/no | No |
| 17 | 1.8 | 6.9 | E | No | 1 | 3/4 | 40 | No/no | No |
| 18 | 1.7 | 5.7 | A | No | 1 | 3/4 | 22 | No/no | Yes |
| 19 | 3.3 | 6.5 | C | Yes | 1 | 3/4 | 28 | No/no | No |
| 20 | 2.1 | 5.0 | E | No | 1 | 3/4 | 29 | No/no | No |
| 21 | 2.1 | 5.0 | A | No | 1 | 3/4 | 40 | No/no | No |
| 22 | 2.2 | 6.0 | E | No | 1 | 3/4 | 68 | No/no | No |
| 23 | 2.0 | 7.0 | C | No | 1 | 4/4 | 24 | No/no | No |
| 24 | 3.0 | 10.5 | E | Yes | 1 | 4/6 | 70 | No/yes | No |
| 25 | 2.0 | 7.9 | C | No | 1 | 3/4 | 35 | No/no | No |
| 26 | 2.1 | 9.0 | C | No | 1 | 3/6 | 38 | No/no | No |
| 27 | 3.7 | 7.0 | E | No | 1 | 4/6 | 57 | Yes/no | No |
ADO-II-AS, Amplatzer Duct Occluder II Additional Size; IB iv, intravenous administration of ibuprofen 1 to 2hours before the procedure; LPA, left pulmonary artery; PDA, patent ductus arteriosus; PT, procedure time from access to sheath removal.
Anatomical classification of ductus arteriosus: “type A”, conical ductus, with prominent aortic ampulla and narrowing at the pulmonary artery end; “type C”, tubular ductus with no constrictions at the pulmonary or aortic artery end; and “type E”, elongated ductus, with restriction at the pulmonary artery end.
A: Angiogram in the left anterior oblique view after release of the Amplatzer Duct Occluder II Additional Size 3/4 device in a 1160g patient with an LPA of 2.2mm in diameter and 6.8 in length; slight protrusion of the device into the LPA was evident. B: Follow-up at 3 months; normal LPA growth was seen with slight nonsignificant color Doppler acceleration, which shows the device in a normal position and with no residual shunt. LPA, left pulmonary artery; PDA, patent ductus arteriosus.
The median length of postprocedural mechanical ventilation was 2 days while that of neonatal ICU admission was 10 days. Three patients died from preterm infant-related comorbidity; there were no deaths related to the PDA or the procedure.
The mean length of postdischarge follow-up of survivors was 35 (4.5-70.0) months. At the last clinic follow-up, all patients showed complete occlusion of the PDA without stenosis of the aortic arch or the LPA and with normal pulmonary pressure. Device protrusion into the LPA causing mild stenosis was seen to resolve in all 4 patients in subsequent follow-up and adequate vessel growth was seen within 3 to 6 months (Figure 1).
Percutaneous treatment of PDA is technically complex in preterm infants due to the size of the material used for the closure, the size of the adjacent vessels, and ductal size and morphology in most preterm infants (moderately sized tubular PDA). Reported complications include residual shunts, embolizations, and obstruction of the aorta and LPA. However, the adverse effects of thoracotomy in preterm infants should not be underestimated. These include pulmonary contusion, bronchial obstruction, diaphragmatic paresis, thoracic deformity, and chylothorax or recurrent laryngeal nerve paralysis.5,6
The ADO-II-AS device is the latest device designed for ductal closure. It has a symmetrical articulated configuration to adjust to the PDA anatomy, as well as a smaller profile for improved accessibility (4 Fr). Although the manufacturer recommends its use for ductal diameters ≤ 4 mm and lengths ≤ 8 mm, 6 patients in our series had a longer ductal length (Table 1) and adequate stabilization of the device was still achieved, showing that this device can be suitable for longer tubular ductus. The ductal diameter is more critical. The highest available waist diameter is 5mm, with a retention disk that is only 1.5mm larger. This leaves little additional anchoring, which is why the maximum ductal diameter should be less than 4mm. No patients in our series had a diameter greater than 4mm; however, considerable care must be taken in those with a borderline diameter of 3.5 to 4.0mm, and our strategy in such patients was to administer an intravenous dose of ibuprofen 1 to 2hours before the procedure to reduce vessel diameter and permit adequate device anchoring. Although these recommendations were followed, 2 embolizations still occurred. We thus stress the importance of using fluoroscopy to visualize constriction of the central portion of the device, which provides ease of mind when the device is released.4
The ADO-II-AS shows a lower rate of protrusion into the aorta and the LPA than its predecessors4,5 and, although there was slight protrusion into the LPA in 4 patients in our series, device removal was not necessary.
Although the cohort comprised very-low-weight patients, there were no complications related to vascular access. In our hands, optimum control of the aortic arch was achieved at recirculation, without the need for arterial access, which minimized the risk of complications.
To conclude, in our experience, percutaneous occlusion of the LPA with the ADO-II-AS is a safe and effective alternative to surgery in carefully selected preterm infants.
.