Implantation of aortic valve prosthesis is the treatment of choice for patients with severe and symptomatic aortic valve stenosis unamenable to surgery. It is thus an alternative to surgery for high-risk patients.1–3
Second generation aortic valve prostheses have been developed to overcome the limitation of the devices currently used, namely, poor affixation and device embolization, perivalvular aortic regurgitation, and atrioventricular conduction disorders. One of these second generation devices is the Engager Aortic Valve (Medtronic Inc, Minneapolis, Minnesota, United States) for transapical implantation. The device can be repositioned and recaptured and it is designed to achieve an anatomical orientation, thereby helping reduce paravalvular regurgitation.
In this article, we describe the first experience reported in Spain of the transapical implantation of this prosthesis in a catheterization laboratory.
Medtronic's biological Engager device has 3 leaflets of bovine pericardium affixed to a nitinol structure. It has a central, self-expanding frame whose proximal part is attached to left ventricular outflow tract. This frame has an external polyester skirt and a support structure and is affixed in 3 places to the aortic part of the central frame (the so-called commissural posts) between which 3 control arms are located, anchored to the base of the sinus of Valsalva. It is available in a size of 26mm (while drafting this article, the 23 mm prosthesis was withdrawn from the market because of elevated residual gradients). The deployment system comprises a 29 Fr introducer and the catheter in which the prosthesis is mounted.
The evaluation of candidates prior to implantation should include a computed tomography angiography to measure the size of the aortic annulus, the sinus of Valsalva, and the sinotubular junction and to identify the best point of access in the chest wall and the optimum working position.4,5
The procedures are performed in the catheterization laboratory under general anesthetic with transesophageal echocardiography by a multidisciplinary team of heart surgeons, interventional cardiologists, an imagining expert, and an anesthetist. An extracorporeal circulation device was made ready in the corridor.
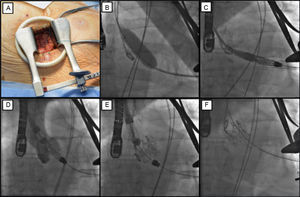
The procedure comprises the following phases (Figure): a minithoracotomy is performed (Figure A) and the apex is punctured by inserting a 6 Fr introducer. A standard guidewire is passed through the aortic valve and then exchanged for a 260-cm Amplatz Super Stiff guidewire. The aortic valvuloplasty is then performed (Figure B). The deployment system is advanced until it has passed through the aortic valve in anterograde position (Figure C). The prosthesis is located in the ascending aorta above the aortic valve plane and rotated to find the correct orientation with fluoroscopic guidance. The control arms are then opened and the device pulled until it lies on the sinuses (Figure D). Angiography and transesophageal echocardiography are used to ensure that the device is appropriately positioned; if the positioning is incorrect, the control arms can be recaptured and the device repositioned. Finally, the commissural posts are released and then the proximal part of the prosthesis, which is now fully deployed (Figures E-F). After the appropriate angiographic, hemodynamic, and echocardiographic evaluations, the introducer is withdrawn from the apex and the ventricular access is closed.
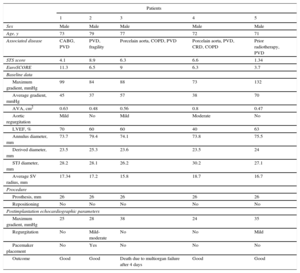
Between February 23 and April 23, 2015, 5 patients were treated. The baseline characteristics and outcomes are shown in the Table. In all cases, the prosthesis was correctly positioned. One patient died on the fourth day after implantation due to refractory multiorgan failure. In this case, no abnormal gradients or significant aortic regurgitation were detected. The remaining patients had a favorable outcome with no further admissions to hospital after a mean follow-up of 221±31 days.
Patient Baseline Characteristics and Outcomes
| Patients | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sex | Male | Male | Male | Male | Male |
| Age, y | 73 | 79 | 77 | 72 | 71 |
| Associated disease | CABG, PVD | PVD, fragility | Porcelain aorta, COPD, PVD | Porcelain aorta, PVD, CRD, COPD | Prior radiotherapy, PVD |
| STS score | 4.1 | 8.9 | 6.3 | 6.6 | 1.34 |
| EuroSCORE | 11.3 | 6.5 | 9 | 6.3 | 3.7 |
| Baseline data | |||||
| Maximum gradient, mmHg | 99 | 84 | 88 | 73 | 132 |
| Average gradient, mmHg | 45 | 37 | 57 | 38 | 70 |
| AVA, cm2 | 0.63 | 0.48 | 0.56 | 0.8 | 0.47 |
| Aortic regurgitation | Mild | No | Mild | Moderate | No |
| LVEF, % | 70 | 60 | 60 | 40 | 63 |
| Annulus diameter, mm | 73.7 | 79.4 | 74.1 | 73.8 | 75.5 |
| Derived diameter, mm | 23.5 | 25.3 | 23.6 | 23.5 | 24 |
| STJ diameter, mm | 28.2 | 28.1 | 26.2 | 30.2 | 27.1 |
| Average SV radius, mm | 17.34 | 17.2 | 15.8 | 18.7 | 16.7 |
| Procedure | |||||
| Prosthesis, mm | 26 | 26 | 26 | 26 | 26 |
| Repositioning | No | No | No | No | No |
| Postimplantation echocardiographic parameters | |||||
| Maximum gradient, mmHg | 25 | 28 | 38 | 24 | 35 |
| Regurgitation | No | Mild-moderate | No | No | Mild |
| Pacemaker placement | No | Yes | No | No | No |
| Outcome | Good | Good | Death due to multiorgan failure after 4 days | Good | Good |
AVA, aortic valve area; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CRD, chronic renal disease; LVEF, left ventricular ejection fraction; PVD, peripheral vascular disease; STJ, sinotubular junction; STS, Society of Thoracic Surgeons; SV, sinus of Valsalva.
This article reports the first series in Spain to date of the Engager valve. One of the potential advantages observed in our series was the feasibility of performing the implantation procedure in a catheterization laboratory with the possibility of using extracorporeal circulation if necessary. Thus, the logistic bottleneck of the availability of an operating theater (in case it is necessary to switch to an open heart procedure) is avoided.
The type of implantation requires close cooperation between the interventional cardiologist, other specialists, and the heart surgeon and is a clear example of converging areas of expertise in certain aspects of these specialties.
None of the patients selected had conditions amenable to surgery although they all had an intermediate surgical risk. The indication for transapical access was the presence of severe peripheral vascular disease. There is no evidence of superiority of this approach compared to others, although the aortic arch is subject to less manipulation in this case.5,6 The success rate for the procedure was very high and comparable to that reported in other series. There were no cases of poor affixation or device recapture.6
In conclusion, our series is the first experience in Spain and demonstrates the feasibility and effectiveness of use of Medtronic's second generation Engager transcatheter device implanted with transapical access in a catheterization laboratory.
CONFLICTS OF INTERESTC. Morís is proctor in Medtronic Corevalve.