Advances in 3-dimensional (3D) image fusion software have enabled easy application of preintervention imaging, including computed tomography (CT) and magnetic resonance imaging (MRI) scans, to create a reliable roadmap for swift manipulation through complex cardiac anatomy.1,2 In an article published in Revista Española de Cardiología, Sandoval et al.3 presented an elegant case report of image fusion of a CT-derived 3D roadmap for aortic stent implantation.
We performed a retrospective review of stent implantation guided by novel image fusion software (VesselNavigator, Philips Healthcare) during the first 9 months since its introduction (Figure). Patient characteristics and catheterization data were reviewed with a focus on the fusion of preintervention imaging and intervention guidance, including structures used for registration, accuracy of 3D reconstruction overlay, and the need for intraprocedural roadmap readjustment.
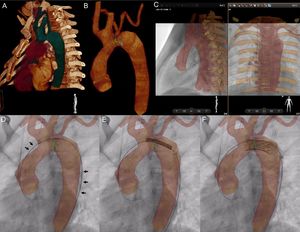
VesselNavigator assisted aortic arch stent implantation in a 12-year-old patient after surgical treatment of interrupted aortic arch (video of the supplementary material). Automatic 3-dimensional (3D) reconstruction was manipulated to outline the aortic arch, proximal branches, and the thoracic aorta (A). A marking ring (green) was placed at the narrowest site, and a marking point (yellow) at the desired proximal end of the stent (B). Further 2 rings (blue) were placed to indicate the origins of the subclavian arteries. Stored fluoroscopy in 2 perpendicular projections, was utilized for manual alignment of the roadmap and live fluoroscopy with bony structures serving as reference structures (C). Introduction of the delivery system led to slight distortion of the anatomy, which is reflected by wire position on the outer curvature of the 3D reconstruction (black arrows, D). The stent was positioned (E) and deployed (F) with the sole 3D roadmap guidance.
VesselNavigator was used in all consecutive patients with available precatherization CT or MRI scans. The 4-step workflow with VesselNavigator, including segmentation, planning, registration and live guidance, has been described elsewhere.4,5 For the registration, stored fluoroscopy images in 2 projections were fused with the 3D roadmap (2D-3D registration) using a combination of internal markers such as bony structures, the cardiac silhouette, areas of calcification, previously implanted devices, and vessel borders visualized with injection of a small amount of contrast medium.
Eighteen patients underwent transcatheter stent implantation for the creation of a “landing zone” prior to percutaneous pulmonary valve implantation (n=7), pulmonary artery stenosis (n=5), aortic coarctation (n=3; video of the supplementary material), aortopulmonary collateral (n=2) and arterial duct (n=1) (Table). A 3D roadmap was created from existing CT (n=17) or MRI (n=1) datasets. For registration, the spine and vertebrae (n=17; 94%), calcifications (n=7, 39%), test angiogram (n=5, 28%) or a previously placed device (n=4, 22%) served as reference points for orientation of the 3D roadmap against live fluoroscopy. Accurate overlay was achieved in all but 2 patients (11%) with an additional 2 patients (11%) requiring intraprocedural realignment due to the introduction of a stiff wire and balloon/stent assembly. All stents were deployed at desired locations with no complications. In 13 patients (72%), the stent was positioned and deployed without prior contrast injection.
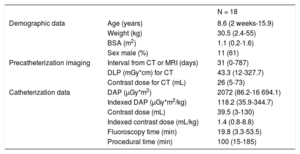
Patient Characteristics and Procedural Data
| N = 18 | ||
|---|---|---|
| Demographic data | Age (years) | 8.6 (2 weeks-15.9) |
| Weight (kg) | 30.5 (2.4-55) | |
| BSA (m2) | 1.1 (0.2-1.6) | |
| Sex male (%) | 11 (61) | |
| Precatheterization imaging | Interval from CT or MRI (days) | 31 (0-787) |
| DLP (mGy*cm) for CT | 43.3 (12-327.7) | |
| Contrast dose for CT (mL) | 26 (5-73) | |
| Catheterization data | DAP (μGy*m2) | 2072 (86.2-16 694.1) |
| Indexed DAP (μGy*m2/kg) | 118.2 (35.9-344.7) | |
| Contrast dose (mL) | 39.5 (3-130) | |
| Indexed contrast dose (mL/kg) | 1.4 (0.8-8.8) | |
| Fluoroscopy time (min) | 19.8 (3.3-53.5) | |
| Procedural time (min) | 100 (15-185) |
BSA, body surface area; CT, computed tomography; DAP, dose area product; DLP, dose length product; MRI, magnetic resonance imaging.
To date and to our knowledge, this is the first series focusing on application of preregistered 3D datasets exclusively for guidance of stent implantation in congenital heart defects. Our initial experience shows that, with modern fusion software, noninvasive 3D images may be easily and effectively reused for positioning and implantation of stents to various locations.2,4,5 A simple registration protocol requires only stored fluoroscopy in 2 projections, akin to the typical setup of isocenter at the beginning of each heart catheterization, and uses a combination of anatomical or iatrogenic landmarks or the injection of a small contrast volume. This 2D-3D registration enabled a shortening of the duration of interventional procedures and a reduction in contrast and radiation exposure.6 In several more recent patients, we felt confident enough to perform stent implantation without prior contrast injection.
To test the reproducibility of these promising individual results, we have initiated a prospective, international registry with contributions from Berlin (Germany), Sejong (South Korea), Mexico City (Mexico), Denver (United States), and Lodz (Poland).
In conclusion, 3D image fusion facilitates precise stent implantation into various anatomical locations. In selected patients, contrast injection may be spared prior to stent implantation.