The onset of atrial fibrillation (AF) when ablation is performed during an electrophysiology study (EPS) hinders and prolongs the procedure. The incidence of AF varies according to the underlying disease and the aggressiveness of the stimulation protocol, but rates of up to 10% have been described.1
Since persistent AF makes it impossible to perform an ablation during an EPS in some substrates, cardioversion (CV) is required to restore sinus rhythm. Pharmacological CV is less effective than electrical CV and also modifies the electrophysiologic properties of tissues, which can cause unsuccessful induction of clinical arrhythmia. As a result, electrical CV has become the method of choice.
Vernakalant is an antiarrhythmic agent indicated in the conversion of recent-onset AF to sinus rhythm. Its antiarrhythmic effect stems from the selective inhibition of potassium channels IKur and IKAch that are specific to the atrial myocardium. Vernakalant causes a frequency- and voltage-dependent block of the sodium channels, also predominantly in the atrium.2 Its rapid conversion of recent-onset AF to sinus rhythm3 and selective action on the atrial myocardium makes it a good candidate for use in the electrophysiology laboratory.
We present our experience of using vernakalant for conversion of AF induced during an EPS. To our knowledge, this is the first prospective series describing the use of vernakalant in this context. Since November 2011, we have administered vernakalant to 19 consecutive patients with AF induced during an EPS.
At the start of the EPS, all patients were in sinus rhythm and AF appeared during catheter manipulation or during the atrial stimulation protocol. After 10 minutes of persistent arrhythmia, we administered vernakalant as the first option in 15 patients (78.9%) and on AF recurrence after effective electrical CV in the other 4 patients (21.1%). We administered the drug by intravenous infusion as recommended in the Summary of Product Characteristics.4
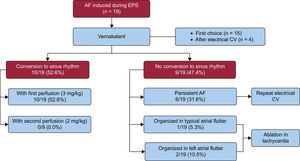
Conversion to sinus rhythm was achieved within 35 minutes of the start of the infusion in 10 patients (52.6%). Mean time to conversion was 9.9min ± 4.0 minutes. Of the 9 remaining patients, 6 (31.6%) remained in AF and underwent electrical CV, which was successful in all patients. The AF organized into sustained typical atrial flutter in 1 patient (5.3%) and into left atrial flutter in 2 (10.5%). Since these arrhythmias matched the patients’ clinical symptoms, we decided to continue the study. As a result, we avoided performing electrical CV in 13 of the 19 patients (68.4%). Atrial fibrillation did not recur in any patients after the administration of vernakalant and the EPS and ablation were successfully completed. Patient response to vernakalant is summarized in the Figure.
At the start of the study, clinical arrhythmia was induced in 11 patients (57.9%): 3 typical nodal reentrant tachycardias, 2 focal atrial tachycardias arising from the right atrium, 2 left atrial flutters, and 4 typical atrial flutters. In all patients, tachycardia could still be induced after administering vernakalant. Ablation was completed successfully in 10 patients and failed in 1 patient with left atrial flutter. The 8 patients (42.1%) with predocumented typical atrial flutter directly received ablation across the cavotricuspid isthmus and we achieved bidirectional conduction block in all patients. After a mean follow-up of 9.6 months ± 11.3 months, clinical arrhythmia recurred only in the 1 patient in whom ablation had failed.
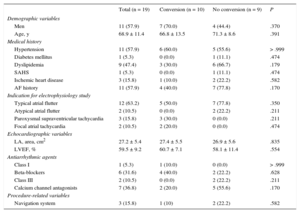
Patient baseline characteristics are shown in the Table, grouped by response to vernakalant. A clinical history of AF (36.4% vs 75%; P = .170) was associated with a lower rate of pharmacological conversion, without attaining statistical significance. Our multivariable analysis found no predictors of lack of response. Previous publications have reported that ventricular dysfunction (left ventricular ejection fraction < 55%) and structural heart disease are predictors of lack of response.5 Our series found no association between these factors and a lower conversion rate. Our results may be explained by the small sample size and low prevalence of these particular factors.
Baseline Characteristics
| Total (n = 19) | Conversion (n = 10) | No conversion (n = 9) | P | |
|---|---|---|---|---|
| Demographic variables | ||||
| Men | 11 (57.9) | 7 (70.0) | 4 (44.4) | .370 |
| Age, y | 68.9 ± 11.4 | 66.8 ± 13.5 | 71.3 ± 8.6 | .391 |
| Medical history | ||||
| Hypertension | 11 (57.9) | 6 (60.0) | 5 (55.6) | > .999 |
| Diabetes mellitus | 1 (5.3) | 0 (0.0) | 1 (11.1) | .474 |
| Dyslipidemia | 9 (47.4) | 3 (30.0) | 6 (66.7) | .179 |
| SAHS | 1 (5.3) | 0 (0.0) | 1 (11.1) | .474 |
| Ischemic heart disease | 3 (15.8) | 1 (10.0) | 2 (22.2) | .582 |
| AF history | 11 (57.9) | 4 (40.0) | 7 (77.8) | .170 |
| Indication for electrophysiology study | ||||
| Typical atrial flutter | 12 (63.2) | 5 (50.0) | 7 (77.8) | .350 |
| Atypical atrial flutter | 2 (10.5) | 0 (0.0) | 2 (22.2) | .211 |
| Paroxysmal supraventricular tachycardia | 3 (15.8) | 3 (30.0) | 0 (0.0) | .211 |
| Focal atrial tachycardia | 2 (10.5) | 2 (20.0) | 0 (0.0) | .474 |
| Echocardiographic variables | ||||
| LA, area, cm2 | 27.2 ± 5.4 | 27.4 ± 5.5 | 26.9 ± 5.6 | .835 |
| LVEF, % | 59.5 ± 9.2 | 60.7 ± 7.1 | 58.1 ± 11.4 | .554 |
| Antiarrhythmic agents | ||||
| Class I | 1 (5.3) | 1 (10.0) | 0 (0.0) | > .999 |
| Beta-blockers | 6 (31.6) | 4 (40.0) | 2 (22.2) | .628 |
| Class III | 2 (10.5) | 0 (0.0) | 2 (22.2) | .211 |
| Calcium channel antagonists | 7 (36.8) | 2 (20.0) | 5 (55.6) | .170 |
| Procedure-related variables | ||||
| Navigation system | 3 (15.8) | 1 (10) | 2 (22.2) | .582 |
AF, atrial fibrillation; LA, left atrium; LVEF, left ventricular ejection fraction; SAHS: Sleep apnea-hypopnea syndrome.
Depending on the presence of a normal distribution, the Student t test or the Mann-Whitney U test was used to compare quantitative variables. The Fisher exact test was used to compare categorical variables.
Data are expressed as no. (%) or mean ± standard deviation.
The patient with typical atrial flutter had an atrioventricular conduction ratio of 1:16. We were able to continue with ablation in this patient without resolving the arrhythmia due to good hemodynamic tolerance and the short duration of the atrial flutter. Another patient had a hypotensive episode with limited chronotropic response despite atropine administration, and required intravenous fluids. We observed no other vernakalant-related complications.
In our experience, vernakalant administration for conversion of AF induced during EPS showed moderate efficacy, similar to results reported in other contexts.3 In addition, we found that vernakalant effectively prevented AF recurrence for the rest of the procedure. Vernakalant exerted its effect without interfering with the induction and ablation of the clinical arrhythmia and showed a good safety profile. The main limitations of our study are the small sample size and lack of comparison with other strategies.
We conclude that in ablation procedures hindered by AF, vernakalant successfully and swiftly converts and stabilizes sinus rhythm without major complications.