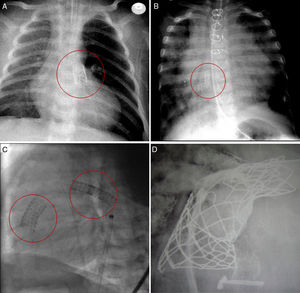
Percutaneous interventions for the treatment of congenital heart disease are constantly improving.1 As surgeons we must be familiar with the advances in this technology for 2 reasons:
- •
The approach to certain heart diseases has changed, particularly complex defects, which require staged percutaneous intervention and surgery.
- •
We will come across stents in the operating room; we must therefore learn how to deal with them and establish patterns or protocols to follow.
We present a series of 105 patients enrolled over 7 consecutive years (2013-2019) in whom previously implanted stents were manipulated during surgery. A previous study2 reviewed the few publications on stents in tetralogy of Fallot,3 patent ductus arteriosis,4 and pulmonary arteries.5
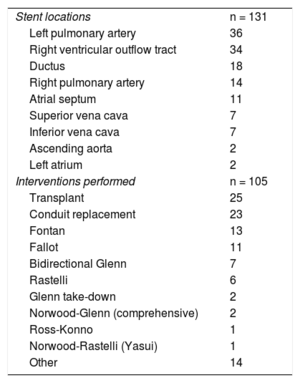
In total, 131 stents were manipulated (table 1) in the following positions: 18 in ductus arteriosus, 34 in the right ventricular outflow tract, 11 in atrial septal defect, 14 in the right pulmonary artery (RPA), 36 in the left pulmonary artery, 7 in the superior vena cava, 7 in the inferior vena cava, 2 in the ascending aorta, and 2 in the left atrium (figure 1). The surgical procedures performed in the 105 patients (table 1) were as follows: 25 transplants, 13 Fontan procedures, 7 Glenn procedures, 2 comprehensive repairs (Norwood+Glenn), 2 Glenn take-downs, 23 conduit replacements (between the right ventricle and the pulmonary arteries), 11 Fallot repairs, 6 Rastelli procedures, 1 Ross-Konno procedure, 1 Yasui procedure (Norwood+Rastelli) and 14 others. Forty-seven of the patients had univentricular physiology.
Stent locations and surgical procedures
| Stent locations | n = 131 |
| Left pulmonary artery | 36 |
| Right ventricular outflow tract | 34 |
| Ductus | 18 |
| Right pulmonary artery | 14 |
| Atrial septum | 11 |
| Superior vena cava | 7 |
| Inferior vena cava | 7 |
| Ascending aorta | 2 |
| Left atrium | 2 |
| Interventions performed | n = 105 |
| Transplant | 25 |
| Conduit replacement | 23 |
| Fontan | 13 |
| Fallot | 11 |
| Bidirectional Glenn | 7 |
| Rastelli | 6 |
| Glenn take-down | 2 |
| Norwood-Glenn (comprehensive) | 2 |
| Ross-Konno | 1 |
| Norwood-Rastelli (Yasui) | 1 |
| Other | 14 |
Depending on the anatomical location, the stents were ligated externally (ductal clip) and partially removed (longitudinal opening and/or trimming the edge) or completely removed (after blunt dissection of the underlying structures). The criteria for partial or complete removal was based on the fragility/consistency of the stented vessel (pulmonary branches, right ventricle) and the surgeon's judgement (according to their experience). In addition, any additional unplanned procedures were also recorded, such as the use of deep hypothermic circulatory arrest (which increases surgical time and morbidity).
The most common anatomical locations stented were the left pulmonary artery and the right ventricular outflow tract. Together these represented two thirds (66%) of all stents. Transplants, conduit replacements and univentricular surgery together made up 80% of the main diagnoses. Unsurprisingly, these were all reinterventions (in some cases, multiple interventions were performed) and in children undergoing staged surgery and percutaneous procedures. Transplant surgery was where most double (or triple) stents were encountered (usually in the left pulmonary artery and superior or inferior vena cava).
The position of the device was not necessarily related to whether it was completely or partially removed. Paradoxically, a left pulmonary artery stent from a Glenn procedure can be cut to accommodate the suture in an extracardiac Fontan procedure (partial removal, easy), while the same patient would require complete removal of a stent in an identical position (plus pericardial patch enlargement of the pulmonary arteries) to allow a transplant to be performed (complete removal, difficult).
In the case of ductal stents, we should differentiate 2 groups: the 10 patients who ultimately would undergo Fallot or Rastelli procedure (external clip, simple procedure) and the other 8 with a hybrid procedure6 and subsequent complex surgery (Norwood-Glenn, Ross-Konno, Yasui, transplant).
Almost half of the patients who underwent surgery in our series had univentricular surgery, in various stages, transplant surgery being the archetypal procedure, involving reinterventions, previous catheterization, univentricular physiology, etc. Surgical planning allows surgeons to anticipate periods of deep hypothermic circulatory arrest if there are 1 or more stents in various positions.
As interventional cardiologists and cardiac surgeons, we must expand our horizons. The challenges we face with more complex patients (multiple surgeries and interventions) provides an opportunity to modify surgical times, offering surgery or catheterization as an alternative or simultaneously (hybrid procedures). One point we can reflect on is that as surgeons we should learn to deal with previously implanted stents, and we can plan the surgical strategy according to the stent location and underlying diagnosis. A stent in a ductus is easy to retrieve in tetralogy of Fallot surgery but difficult to retrieve in a hybrid procedure; a device in the left pulmonary artery is easy to deal with in a conduit replacement but complex if performing a Fontan for subsequent transplant. The data analyzed in this study show us that complex heart defects (eg, reinterventions, univentricular heart, transplant) often involve intracardiac stents. As surgeons acquire experience in their management during subsequent surgery, interventionalists may be encouraged to offer stenting in future patients, either alone or combined with surgery (hybrid procedures). As both specialties evolve (interventional cardiology and cardiac surgery) we must constantly review our processes.