Transcatheter aortic valve implantation (TAVI) is a technique that was first introduced more than 10 years ago and has since become established as an alternative to surgery in the treatment of severe symptomatic aortic valve stenosis. Since the pioneering valve prosthesis implantation of Dr. A. Cribier in 2002, it is estimated that more than 200000 TAVI procedures have been performed throughout the world. The first studies of TAVI included inoperable patients and those at high surgical risk and compared the outcomes with medical treatment and surgery, respectively. In the studies of patients with high surgical risk, TAVI appeared to be a superior alternative to surgery1; these findings have since been confirmed in numerous registries. The initial studies also highlighted the weaknesses of the technique when compared with surgery. For example, these studies detected increased incidences of vascular complications, cerebrovascular accidents (CVAs), need for pacemaker implantation, and the presence of significant aortic regurgitation after implantation. These weaknesses have served as a guide for technical improvements to the initial platforms and the design of new prostheses (Figure). Examples of these improvements include reduction of the deployment system size to reduce the incidence of vascular complications and disabling CVAs. Another important technical advance has been the addition of an outer skirt to the stent to improve prosthesis sealing and thus prevent moderate-severe perivalvular leakage. This is crucial because the presence of such leaks is an unfavorable prognostic factor. For example, in the case of the Edwards valve, the incidence of moderate-severe paravalvular leakage has decreased from between 12% and 24% to 2% with the latest generation of this valve. Another important aspect, above all if the technique is to be used in patients with lower risk, is the rate of pacemaker implantation, which varies between 12% and 30% according to the type of valve used (Table).1–5
Examples of latest-generation valves. A, Edwards SAPIEN 3 valve (Edwards Lifesciences; Irvine, California, United States). B, CoreValve Evolut R valve (Medtronic; Minneapolis, Minnesota, United States). C, Lotus valve (Boston Scientific; Natick, Massachusetts, United States). D, ACCURATE neo valve (Symetis; Ecublens, Switzerland).
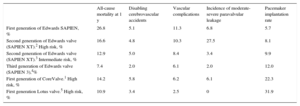
Rates of All-cause Mortality, Disabling Cerebrovascular Accidents, Major Vascular Complications, and Pacemakers at 1 Year of Follow-up for Different Generations of Edwards, CoreValve, and Lotus Prostheses
| All-cause mortality at 1 y | Disabling cerebrovascular accidents | Vascular complications | Incidence of moderate-severe paravalvular leakage | Pacemaker implantation rate | |
|---|---|---|---|---|---|
| First generation of Edwards SAPIEN, % | 26.8 | 5.1 | 11.3 | 6.8 | 5.7 |
| Second generation of Edwards valve (SAPIEN XT).2 High risk, % | 16.6 | 4.8 | 10.3 | 27.5 | 8.1 |
| Second generation of Edwards valve (SAPIEN XT).3 Intermediate risk, % | 12.9 | 5.0 | 8.4 | 3.4 | 9.9 |
| Third generation of Edwards valve (SAPIEN 3),4% | 7.4 | 2.0 | 6.1 | 2.0 | 12.0 |
| First generation of CoreValve.1 High risk, % | 14.2 | 5.8 | 6.2 | 6.1 | 22.3 |
| First generation Lotus valve.5 High risk, % | 10.9 | 3.4 | 2.5 | 0 | 31.9 |
The first randomized study published in 2015 was the NOTION study.2 This study compared the CoreValve prosthesis (Medtronic; Minneapolis, Minnesota, United States) with surgery in 280 patients of low-intermediate risk aged over 70 years (all comers). The Society of Thoracic Surgeons (STS) score was approximately 3%. The primary outcome measure was a composite of all-cause death, CVA, or myocardial infarction at 1 year and was similar in both treatment groups.
In 2016, 3 important studies were published. The PARTNER 2 cohort A study3 had a randomized noninferiority design and enrolled 2032 patients with severe symptomatic aortic stenosis, stratified by whether or not transfemoral access was feasible. Patients with feasible femoral access (n = 1550) were randomized to TAVI with the Edwards XT prosthesis (Edwards Lifesciences; Irvine, California, United States) or conventional surgery. Similarly, patients without feasible femoral access (n = 482) were randomized to TAVI or surgery, with TAVI performed via transapical or transaortic access. The primary outcome measure of the study was all-cause mortality or CVA at 2 years of follow-up and was similar in both groups. However, when the cohort implanted with transfemoral access was analyzed alone, TAVI was associated with a decreased mortality and rate of CVA compared with surgery; these decreases were seen within the first few months and persisted for up to 2 years of follow-up. These encouraging results showed that TAVI is a reasonable or even superior alternative to surgery in patients with moderate risk, even though the latest generation of Edwards valves was not used in the study. In the same year, the results of a registry with 1077 patients of intermediate risk were published.4 These patients received a latest-generation Edwards valve, the SAPIEN 3, and a transfemoral approach was used in more than 85%. The outcomes at 1 year in terms of all-cause mortality, rate of CVA, and rate of moderate-severe aortic regurgitation were compared with the surgery cohort of the PARTNER 2 A study. When the propensity score was calculated, not only was the TAVI group noninferior, but it was actually superior to surgery.
Finally, a meta-analysis of 4 randomized studies was published (PARTNER 1 A, US CoreValve, NOTION, and PARTNER 2 A, including a total of 3806 patients).6 The meta-analysis found a 13% relative risk reduction for all-cause mortality at 2 years in the TAVI-treated group when compared with surgery. Furthermore, these results were consistent for patients with intermediate and high risk. Still unavailable are the results of the randomized studies of patients with intermediate risk such as the SURTAVI (NCT01586910) trial and with low risk such as the PARTNER III (NCT02675114) and the Evolute Low Risk (NCT02701283) trials, which have been approved by the United States Food and Drug Administration.
In short, the highlights of 2016 in the treatment of severe symptomatic aortic valve stenosis were the findings of the first randomized studies in patients with intermediate surgical risk because these results have helped to establish this technique as an alternative to surgery in this group of patients. Although important questions remain to be answered, such as the long-term durability of the prosthesis, thanks to the findings of these studies and the improvements in both valve techniques and operator skill, we are now in a position to begin studies of low-risk patients.