The last year saw the publication of various noteworthy studies in the field of percutaneous catheter ablation aimed at reducing procedure-related complications by refining the perioperative approach, optimizing patient selection, demonstrating the viability and benefit of the invasive approach for unconventional substrates, and improving arrhythmia recurrence-free survival outcomes.
Regarding the perioperative management of atrial fibrillation (AF) ablation, the evidence supports the strategy of not interrupting anticoagulant treatment with vitamin K antagonists during the procedures. In addition, more and more patients presenting to electrophysiology laboratories are under treatment with novel oral anticoagulants (NOACs). Several studies have been launched to determine the safety of the uninterrupted approach for these patients. After the VENTURE-AF study,1 which showed the plausibility of ablation in patients under uninterrupted treatment with rivaroxaban, the results have been published of the randomized, multicenter trial RECIRCUIT,2 a study that assessed the safety of uninterrupted dabigatran versus warfarin during ablation procedures. With a primary end point of the rate of major bleeding events 2 months after ablation, dabigatran was clearly superior to warfarin (1.6% vs 6.9%, P < .001). There was only one thromboembolic event (secondary end point) in the warfarin group. The rates of minor bleeding were similar in the 2 groups. These data support the use of dabigatran for uninterrupted treatment during ablation procedures.
According to data from the European ablation registry, the profile of patients undergoing AF ablation procedures in our setting is still that of a relatively young patient (average age, 59 years) with little comorbidity and normal ventricular function (IQR 55-65). Because very few of these patients have advanced heart disease (<1%), the results of the CASTLE-AF multicenter randomized clinical trial,3 recently presented at the European Congress of Cardiology 2017, are surprising. This study compares the efficacy of the AF ablation procedure with that of standard treatment during the follow-up of 397 patients with severe heart failure (left ventricular ejection fraction < 35%, New York Heart Association functional class > II) who have an implantable automatic cardioverter-defibrillator (ICD) capable of monitoring the arrhythmia burden. The novelty of this study is that the primary end point is death from any cause and hospitalization for HF. The primary end point occurred in 28.5% of the ablation group and in 44.6% of the control group after a mean follow-up of 37.8 months (relative risk reduction = 38%; hazard ratio [HR] = 0.62, 95% confidence interval [95%CI], 0.43-0.87). This study shows for the first time that AF ablation not only improves patients’ symptoms, but also reduces morbidity and mortality in the mid-to-long term. Although they are striking results, because the patients involved were highly selected and do not reflect the typical patient profile, the results must be interpreted with caution.
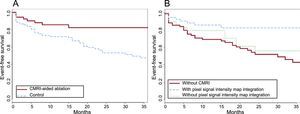
Characterization of the arrhythmic substrate of patients with ventricular tachycardia (VT) and underlying heart disease allows successful ablation without the need for tachycardia mapping and reduces the rate of sustained VT recurrences versus clinical VT ablation alone during follow-up in patients with well-tolerated arrhythmia. Advances in imaging techniques, particularly delayed enhancement magnetic resonance imaging (DE-MRI) characterization of the substrate, have helped to improve ablation outcomes. The study by Andreu et al.4 showed a reduction in the short- and long-term rates of VT recurrence with a substrate ablation strategy guided by the fibrosis detected on 3-T MRI, which identifies the regions of heterogeneity of the fibrotic scar contributing to the formation of the reentry circuits. The strategy supported by DE-MRI had an 18.5% rate of VT recurrence versus 43.8% for the control group, comprising those who could not undergo MR or whose MRI was not of good quality (Figure). In multivariable analysis, the guided ablation strategy was an independent predictor of recurrence (HR = 0.48; 95%CI, 0.24-0.96).
Kaplan-Meier curves for the VT recurrence- or ICD therapy-free survival of patients undergoing VT ablation. A: Comparison of patients managed using a CMRI-aided ablation strategy with controls. B: The same comparison placing in the control group patients with available CMRI pixel signal intensity maps but with integration performed with the navigation system. CMRI, cardiac magnetic resonance imaging; ICD, implantable cardioverter-defibrillator; VT, ventricular tachycardia. Reproduced with the permission of Andreu et al.4
In addition, a multicenter and randomized study5 showed no benefit from substrate ablation in patients with an ICD and unstable VT; time until first recurrence of VT/VF was used as the primary end point. Recurrence was observed in 51% of the patients with ablation and ICD versus 48.6% of the patients with ICD alone in a mean follow-up of 2.2 years. Ablation was still associated with a reduction in the total number of VF/VT episodes with or without therapy.
A relevant novelty is radiofrequency ablation in Brugada syndrome. There were few therapeutic options for patients with Brugada syndrome and ventricular arrhythmias. Ablation in this context, although previously reported, was limited to a small series. The recent study by Pappone et al.6 included 135 symptomatic ICD carriers with spontaneous or inducible VT in the electrophysiological study. For better epicardial characterization of the substrate, the mapping was performed with ajmaline infusion. The objective was to identify and eliminate all delayed and fragmented potentials in the right ventricular epicardium in order to normalize the electrocardiographic pattern and ensure that no arrhythmias were induced in the electrophysiological study. After a mean follow-up of 10 months, electrocardiographic normalization and the absence of spontaneous or inducible ventricular arrhythmias persisted in 98.5% of the patients.
In summary, research in the field of percutaneous ablation during 2017 has highlighted the safety of uninterrupted intraprocedural treatment with NOACs, an improvement in mortality and hospitalizations for patients with HF and AF who undergo ablation, and the importance of the integration of imaging techniques with navigation systems to reduce the ventricular arrhythmia burden. The good results with the substrate approach in patients with ventricular arrhythmias and Brugada syndrome open the door to an expansion of the therapeutic options in complex cases and, if these results are maintained during long-term follow-up, they may help to alter the prognosis and the therapeutic approach to this condition.