Recent data on catheter ablation approaches and outcomes have provided new clinical perspectives on our main goal of successful arrhythmia termination and lack of recurrences during follow-up.
The results are especially interesting in atrial fibrillation (AF) ablation; this substrate has progressively increased over the last 15 years and is currently the leading procedure in many electrophysiology laboratories in developed countries. The latter highlights the relevance of single-shot approaches vs conventional radiofrequency delivery as an attempt to decrease procedure duration while maintaining the efficacy achieved by conventional point-by-point ablation. Thus, the results of the randomized and multicenter FIRE and ICE trial have shown that cryoballoon ablation was not inferior to radiofrequency ablation with regard to documented recurrence of AF, documented occurrence of atrial flutter or atrial tachycardia, prescription of antiarrhythmic drugs (class I or III), or repeat ablation.1 Procedure duration was significantly shorter in the cryoablation group than in the radiofrequency group (124.4 ± 39.0 vs 140.9 ± 54.9minutes, respectively), although fluoroscopy time was significantly longer in the cryoablation group (21.7 ± 13.9 vs 16.6 ± 17.8minutes). Complication rates did not differ between the 2 groups, although 1 case of esophageal ulcer was reported in the cryoablation group. The study only included paroxysmal AF patients, which precluded extrapolating such results to more complex substrates such as persistent AF. Success rates were ≈ 65% in both groups after a mean follow-up of 1.5 years, which is close to what has been reported in the presence of continuous rhythm monitoring, when experienced operators perform both techniques.2
Despite ≈ 70% freedom from AF after 1-year of follow-up, the established conventional approach of pulmonary vein isolation (PVI) during AF ablation still shows an important lack of specificity, which precludes increasing efficacy. The latter becomes more relevant with persistent AF, in which the success rate may decrease to 30% after 5 years of follow-up if subsequent procedures upon recurrences are not performed. Data from mechanistically based approaches have been shown to be promising in persistent AF with success rates of up to 77.8% after a median follow-up period of 2.4 years. The main aim of these approaches is to target specific atrial areas that may host rapid reentrant activity. However, it requires processing complex patterns of propagation occurring during AF by means of modern tools and computational analysis that have not been released to the scientific community for proper evaluation. This has generated many concerns among conventional electrophysiology laboratories, especially after the publication of completely different results from the multicenter OASIS trial, which showed poor success rates (14% free of AF/atrial tachycardia-free of antiarrhythmic drugs at 1-year follow-up) using the focal impulse and rotor modulation (FIRM)-guided ablation. However, this work, led by Natale et al. has been recently retracted by the editorial board of the Journal of the American College of Cardiology, due to nondisclosed deviation from a random allocation of participants to treatments across sites. This retraction further sharpens current confusion in the field until new trials are properly conducted.
Another very recent multicenter and randomized trial aimed to compare amiodarone vs AF ablation in challenging substrates, such as persistent AF in patients with left ventricular ejection fraction ≤40% and a dual chamber implantable cardioverter defibrillator (ICD) or cardiac resynchronization device (AATAC trial).3 The ablation strategy went beyond PVI alone and included ablation of extensive areas of the left atrium plus isolation of the superior vena cava in certain cases and redo procedures if necessary. The results showed that catheter ablation was superior to amiodarone in achieving freedom from AF during long-term follow-up. More stunning was that catheter ablation reduced unplanned hospitalizations and overall mortality, which needs to be confirmed in other trials.
Another complex substrate with recent advances leading to clinical implications is ventricular tachycardia (VT) ablation in patients with underlying coronary artery disease and recurrent VT. The prospective, nonrandomized and multicenter Post-Approval THERMOCOOL VT trial has shown that VT ablation significantly reduced sustained monomorphic VT recurrences by 62% at the 6-month follow-up. Moreover, 41% of patients were free from VT after a 3-year follow-up.4 This outcome translated into a statistically significant decrease in hospitalizations, ICD shocks, and amiodarone use. The ablation approach to identify target sites was left to the investigators’ criteria, while recommending activation and entrainment mapping during VT to guide the ablation sites. Substrate characterization by voltage mapping, identification of split or late potentials and/or pace maps with long stimulus to QRS intervals, in which the QRS mimics the target VT, were recommended when VT was intolerable.
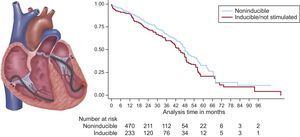
Another step forward in VT ablation came from the VANISH trial,5 which was a multicenter, randomized study aiming to compare catheter ablation with continuation of baseline antiarrhythmic medications or escalated antiarrhythmic drug therapy in patients with prior myocardial infarction, ICD, and recurrent VT. Patients within the antiarrhythmic drug group were treated with amiodarone or amiodarone plus mexiletine. The primary outcome was a composite of death or VT storm or appropriate ICD shock after a 30-day treatment period, including as secondary outcomes all-cause mortality and hospital admissions for cardiac causes, among others. The ablation strategy was similar to that used in the postapproval THERMOCOOL VT trial. Catheter ablation demonstrated to be more effective than antiarrhythmic drug therapy in reducing the primary endpoint after 27.9 ± 17.1 months of follow-up, although mortality did not significantly differ between groups. With respect to mortality, it is likely that this study was underpowered. Large registries indicate that VT ablation, especially in postinfarction patients, appears to reduce mortality if successfully performed (Figure).6
Survival in patients with ablated VT. In patients who have experienced a myocardial infarction, catheter ablation can render ventricular tachycardia (VT) noninducible and thereby reduce mortality and VT recurrence. The left panel illustrates catheters positioned in the right ventricle for programmed stimulation and in the left ventricle for ablation of VT within scar tissue. The right panel shows the Kaplan-Meier survival curve for those with noninducible VT compared with those with inducible VT or who did not undergo programmed stimulation. Noninducibility postablation was independently associated with lower mortality (log-rank, P = .02). Reproduced with permission from Yokokawa et al.6
The best of catheter ablation in 2016 provides the first evidence of improved outcomes with decreased hospitalizations and possibly also mortality after AF ablation in heart failure patients, and reduced death or VT storms or appropriate ICD shocks after VT ablation in patients with an infarct-related substrate. New imaging and mapping techniques for both substrates may further improve such outcomes and hopefully improve long-term success in the near future.