There is histological evidence that drug-eluting stents are associated with delayed endothelialization and a persistent inflammatory state. Moreover, clusters of inflammatory cells have been observed on luminal surfaces by scanning electron microscopy. With the aim of quantifying this inflammatory response, we implanted one bare-metal stent and two drug-eluting stents containing different doses of vinblastine embedded in the same polymer into the coronary arteries of 12 domestic pigs. The density of inflammatory cells in a representative area (100x100μm) was quantified at 3 and 7 days. Endothelialization was more complete in bare-metal stents than in drug-eluting stents at both 3 days (P=.016) and 7 days (P=.0001). The degree of inflammation induced by the drug-eluting stents was higher than that induced by the bare-metal stents at both 3 days (11.8±3.5% vs. 4.5±2%; P=.001) and 7 days (26.3±4.4% vs. 1.2±1.5%; P=.0001). In addition, the time sequence was inverted: the inflammatory response increased over time with the drug-eluting stents, while the opposite occurred with the bare-metal stents.
Keywords
Coronary drug-eluting stents (DES) have proved effective in reducing restenosis.1,2 However, DES are also associated with delayed endothelialization and persistent inflammatory states which may be due to either the antiproliferative effects of the drugs used or to the polymer used for controlled release.3,4,5,6 Both phenomena have been associated with stent thrombosis. the most serious complication of these devices and one which can occur in even very late phases.4 The data used in these studies came from the histological analysis of animal parts and human autopsies. Scanning electron microscopy (SEM), which permits observation of inflammatory cells in the arterial wall and the stent, has frequently been used to analyze stent endothelialization. The aim of this study was to describe a method for the analysis of superficial (luminal) inflammation induced by coronary stents which allows both the extent and evolution of inflammation to be assessed in different types of device.
MethodsThe experimental procedures were performed in 12 domestic pigs (25±3kg), following the general guidelines for the protection of experimental animals (Directive 86/609/EEC RD1201/2005) and under the supervision of the center's bioethics committee.
Sedation, analgesia, anesthesia, and endovascular procedures have been described previously.7 One bare-metal stent (BMS, Apollo 3.5® x 18mm, Iberhospitex SA, Spain) and two drug-eluting stents using different doses of vinblastine (DES1, 0.18 x μg/mm2 and DES2, 0.36μg/mm2) embedded in the same polymer (Polymer P5®, Iberhospitex SA-Palau Pharma SA, Spain) were randomly implanted so that the distribution of stents in each of the three coronary arteries (left anterior descending, circumflex and right coronary) was the same. Implantation pressure was adjusted to obtain a 10% oversizing.
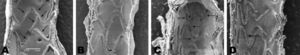
Euthanasia of the animals at 3 (n=6) and 7 days (n=6) after implantation has been described previously.7 The procedures used in the SEM study followed the methodology described by Farb et al.8 The luminal surfaces of the hemisection were examined by JSM-6480 LV scanning electron microscope (JEOL, Japan). Each hemisection was digitally photographed and endothelized surfaces were analyzed at 25X magnification. Detailed imaging (150-250X magnification) was used to differentiate endothelial cells from fibrin formations or other cell types. The percentage of endothelialization was determined by measuring the total area of the part, corrected by the metal-to-artery ratio (14%) and the non-endothelized metallic area using the following formula: %=100 x [1 - (area not endothelized / total metallic area)]. Between 3 and 5 representative areas (100 x 100μm) of arterial wall adjacent to the stent were selected (Figure 1) to study the density of inflammatory cells, which are larger than other blood cells. After processing the image using automatic threshold determination with ImageJ-NIH Image 1.4 software (National Institutes of Health, USA), the density of leukocytes in the area analyzed was quantified (percentage of total area occupied by inflammatory cells).
Figure 1. Image processing for analysis of inflammation. A: inflammatory response to the stent in the region adjacent to the wall. B: gray-level threshold processing and selection of a representative 100 x 100μm area (red area). C: detail of the size and morphology of leukocytes (spherical, ∼ 10μm, red arrow), red blood cells (biconcave, ∼ 5μm, blue arrow) and platelets (small dots <1μm, green arrow).
Data are presented as means±standard deviation. Differences between means were analyzed using the Student t test and correlations using Pearson's r2.
Supplementary material associated with this section can be found in the online version of this article.
ResultsEndothelialization was more complete in BMS than DES at both 3 days (82%±18% vs. 28%±14%, P=.016) and 7 days (97%±3% versus 44%±15%, P=.0001) (Figure 2). No difference in endothelialization was observed between the two DES, between the different arteries (anterior descending, circumflex and right coronary artery) or according to the stent / artery ratio (BMS, 1.17±0.1; DES1, 1.07±0.07; DES2, 1.13±0.16, P=0.4).
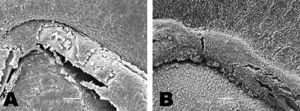
Figure 2. A and B: endothelialization of the bare-metal stent. A: at 3 days, partial endothelialization of the stent; bare areas (arrows). B: after 7 days, complete endothelialization of the stent. C and D: endothelialization of the drug-eluting stent. C: after 3 days, bare areas (arrows) and the rest covered with fibrin. D: after 7 days, irregular endothelialization; presence of giant cells (arrows).
BMS showed a lower density of inflammatory cells than DES at both 3 days (4.5%±2% vs 11.8%±3.5%; P=.001) and 7 days (1.2%±1.5% vs 26.3%±4.4%; P=.0001) (Figure 3). No statistically significant differences were observed between the two DES. The density of inflammatory cells decreased significantly over time with BMS (4.5%±2% versus 1.2%±1.5%; P=.0001). However, the opposite was true for DES, which showed a significant increase after 7 days (11.8%±3.5% vs. 26.3%±4.4%; P=.0001).
Figure 3. Degree of inflammation. A: bare-metal stent at 3 days, small number of inflammatory cells (wall and stent); partial endothelialization of the stent; areas with fibrin. B: drug-eluting stent at 7 days, intense inflammatory reaction around the stent; fibrin and platelets covering in the stent.
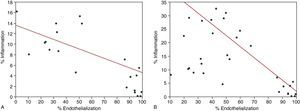
Inverse correlations were observed between the degree of inflammation and the percentage of endothelialization, with r2 values of -0.4 (P<.011) at 3 days and -0.84 (P<.0001) at 7 days (Figure 4).
Figure 4. Correlation between the degree of endothelialization and inflammation. Results at 3 days (A) and 7 days (B).
DiscussionThe recent consensus document9 on preclinical analysis of DES indicated that semi-quantitative assessment of inflammation induced was one of the most important parameters to take into account. The method described here shows little variability and provides results on a quantitative scale, thereby allowing for more detailed comparison between the responses obtained with different devices.
Differences in long-term response observed in a porcine model between two first-generation DES were published recently.6 Parameters differentiating between the two stents were in-depth inflammatory response and fibrin deposition. Although the authors acknowledged the difficulty of extrapolating the findings to the clinical field, the fact remains that the findings were undesirable. The analysis of luminal inflammation completes the study of the response to DES via phenomena occurring at the stent-lumen interface, phenomena which are essential to subsequent endothelialization.5 In fact, we observed a significant inverse correlation between the intensity of the inflammatory response and the percentage of endothelized stent surface.
A noteworthy finding was the temporal pattern of inflammation, with decreasing inflammatory response being observed in BMS and an increasing response in DES. The release of the drug may play an important part in these results, as it is a short-term analysis. Nevertheless, the persistence of these phenomena over the very long term in other studies5,6,9 seems to confirm the causal role of the polymers used. The possible role of the P5® polymer in the increase in inflammatory response cannot be determined with the data presented here, but preliminary findings do not provide evidence for such a relationship.10
The most notable limitation of the study is the difficulty inherent in extrapolating (young and healthy) animal model data to sick humans, although it is recommended by the consensus document.9 The use of sick (diabetic, hyperlipidemic, or induced genetic deficiency) animal models is being validated but is not yet recommended. The fact that these observations were not correlated with findings using other analytic techniques (histology) can be seen as another limitation. However, we believe that the method described supplements information obtained by other means, rather than replacing it. The role that other antiproliferative drugs may play in inflammatory response remains to be defined.
In conclusion, the vinblastine-eluting stents used in this study produced a greater degree of inflammation than bare-metal stents. In addition, the time sequence was inverted: inflammatory response increased over time with the DES, while the opposite was true for bare-metal stents. There was a significant correlation between observed inflammatory response and coronary stent endothelialization.
FundingSupported by an unrestricted grant from Cordynamic-Iberhospitex, S.A. (Barcelona, Spain).
Conflicts of interestNone declared.
Appendix. Supplementary MaterialSupplementary material associated with this article can be found in the online version available at doi:10.1016/j.rec.2010.04.001.
Appendix. Supplementary dataReceived 23 February 2010
Accepted 24 April 2010
Corresponding author: Servicio de Cardiología Intervencionista, Hospital de León, Altos de Nava, s/n. 24008 León, Spain. aperez@secardiologia.es