Recently, percutaneous aortic valve replacement has emerged as a therapeutic option for patients with severe symptomatic aortic stenosis and a high surgical risk. We report our initial experience in four patients with percutaneous implantation of a CoreValve aortic prosthesis to treat aortic bioprosthesis dysfunction involving aortic stenosis or regurgitation. In-hospital and medium-term outcomes were analyzed. The procedure was performed under local anesthesia and guided by angiography. The prosthesis was implanted successfully in all patients, although a second prosthesis was required in one case because the first was positioned too high. There were no major complications. After a mean follow-up of 7 months (SD, 4.7), all patients remained asymptomatic.
Keywords
The worldwide incidence of aortic valve stenosis has been increasing exponentially as life expectancy rises.1,2 Aortic valve replacement is the standard treatment, and biological prostheses have been the treatment of choice for patients of advanced age or with comorbidities.3 These cardiac biological prostheses have a short life-span, which may be further shortened by various degenerative processes,4 and repeat procedures involve considerable risk to this patient group.5
Percutaneous aortic valve replacement is an alternative for the treatment of severe symptomatic aortic valve stenosis at high surgical risk.6,7,8,9,10 Patients with aortic bioprosthesis dysfunction can be considered a subgroup at high risk. We present our initial experience in treating aortic bioprosthesis dysfunction (due to stenosis or regurgitation) by percutaneous implantation of the CoreValve aortic valve prosthesis.
MethodsIn 2008 an assessment and percutaneous CoreValve aortic prosthesis implantation program was put into practice for high-risk surgical or elderly (>80 years) patients who refused valve replacement surgery. Patients were selected in accordance with the recommendations of the various scientific societies11 and the anatomic criteria required for percutaneous implantation.6
Between April 2008 and November 2009, 69 patients were consecutively treated with the CoreValve percutaneous aortic prosthesis due to severe symptomatic aortic valve disease at high surgical risk. Aortic bioprosthesis dysfunction was present in 4 patients: 2 with severe aortic regurgitation and 2 with aortic stenosis.
All patients were clinically assessed, and surgical risk was estimated by the logistic EuroSCORE. In addition, all patients underwent echocardiography, coronary angiography, aortography of the aortic root, and angiography of the iliofemoral axis.
The large CoreValve prosthesis was selected for a surgical bioprosthesis with a diameter of 23mm or larger and the small prosthesis for a diameter smaller than 23mm.
Description of the Device and ProcedureThe CoreValve aortic prosthesis is a biological prosthetic trileaflet valve of porcine pericardium, fitted and sutured onto a self-expanding nitinol structure. There are 2 valve sizes: small (26mm in the portion inserted into the native annulus) and large (29mm). The length of the prosthesis is 50 mm.
ProcedureThe procedures were performed under local anesthesia with superficial sedation. In all 4patients, access was through the femoral artery using a 18 French introducer and the femoral puncture was closed with the Prostar XL® percutaneous device. The aortic prosthesis was expanded under angiographic control. In the case of bioprosthesis dysfunction with aortic regurgitation, valvuloplasty was not performed before the prosthesis was implanted. No difficulties were experienced while advancing the CoreValve prosthesis through the surgical bioprosthesis. Echocardiographic follow-up was performed at 72h.
The procedure was considered successful if the normal functioning prosthesis was correctly implanted and there was no in-hospital mortality.
ResultsThe mean age of the patients with bioprosthesis dysfunction was 77.2 (SD, 13.5; range, 60–89) years; the mean logistic EuroSCORE was 33.7% (SD, 24.6%; range, 7.14%–63.9%). Table 1 summarizes the baseline characteristics. The 2 patients with prosthetic dysfunction caused by severe stenosis had a Carpentier-Edwards bioprosthesis (19 and 27mm, respectively). In the patients with aortic regurgitation, the aortic bioprosthesis was frameless and with radiolucent ring: 23-mm Intact (Medtronic) and 21-mm Mosaic (Medtronic).
Table 1. Baseline Characteristics of Patients with Degenerated Bioprosthesis *
| 1 | 2 | 3 | 4 | |
| Age, years | 60 | 87 | 73 | 89 |
| Sex | Male | Female | Female | Female |
| Logistic EuroSCORE, % | 7.14 | 41.92 | 22.1 | 63.9 |
| Coronary disease | Yes | Yes | Yes | Yes |
| Dyspnea, NYHA functional class | IV | IV | IV | IV |
| Ejection fraction, % | 66 | 68 | 57 | 75 |
| Bioprosthesis | 27-mm C-E | 19-mm C-E | 23-mm intact | 21-mm mosaic |
| Degree of calcification | Mild | Moderate | Moderate | Mild |
| Duration of bioprosthesis, y | 3 | 11 | 14 | 9 |
| Dysfunction | Stenosis | Stenosis | Regurgitation | Regurgitation |
| Comorbidity | Cirrhosis of the liver | Rheumatoid arthritis |
Abbreviations: C-E, Carpentier-Edwards; NYHA, New York Heart Association.
* Patient 1 presented as comorbidity a cirrhosis of the liver complicated with portal hypertension. Patient 3 presented as comorbidity a rheumatoid arthritis with chest deformation.
The CoreValve aortic prosthesis was successfully implanted in all 4 patients. Small prostheses were used in 2 patients and large prostheses in 2. The mean procedure time was 117 (11.5)min and the mean aortic prosthesis expansion time, 7.7 (2.8)min.
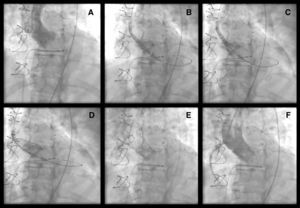
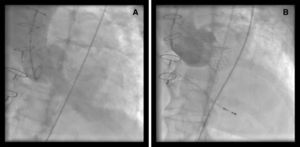
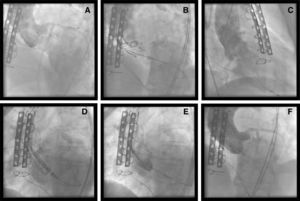
CoreValve aortic prosthesis expansion was easier in patients with a radio-opaque ring because the bioprosthesis ring was visualized under radioscopy (Figure 1), unlike the frameless bioprosthesis (Figure 2). Following implantation of the CoreValve aortic prosthesis, hemodynamic improvement was obtained and the aortic gradient disappeared. One patient required a second prosthesis during the same procedure because the first one remained in the supra-annular position, leading to severe aortic regurgitation (Figure 3). Postprocedure aortic regurgitation grade was trivial in 2 patients and mild in the other 2. There were no cardiovascular or cerebrovascular complications.
Figure 1. (A) Carpentier-Edwards bioprosthesis dysfunction due to severe stenosis. (B–D) Different time points during CoreValve prosthesis expansion. (E–F) Angiographic outcome after implantation.
Figure 2. Dysfunctioning frameless aortic bioprosthesis. (A) Severe aortic regurgitation. (B) Angiographic outcome after implantation.
Figure 3. Degenerated biological prosthesis. (A) Severe aortic regurgitation. (B) Prosthesis expansion. (C) Severe aortic regurgitation after high implantation of the prosthesis. (D–E) Implantation of second CoreValve prosthesis. (F) Angiographic outcome.
The maximum echocardiographic aortic gradient decreased from 66.7 (25.7) mmHg to 24 (7.4)mmHg and the mean, from 44 (22.7) mmHg to 14.7 (5.6)mmHg.
One patient required a pacemaker 4 days after the procedure due to paroxysmal atrioventricular block on the third day.
The in-hospital stay was 5.2 days (SD, 0.9; range, 4–6). By the time of discharge, NT-proBNP had dropped from 5308 to 3418pg/ml and the patients presented clinical improvement (from New York Heart Association functional class IV to I–II).
After a mean follow-up of 7 months (SD, 4.7; range, 4–14), all patients remained asymptomatic.
DiscussionPercutaneous implantation of the CoreValve aortic prosthesis represents a new option for the treatment of severe aortic stenosis in patients at high surgical risk.6,7,8,9,10 Once the initial learning curve was overcome and excellent periprocedure and medium-term outcomes were observed, however, the use of the bioprosthesis has been extended to other indications not originally considered. One is aortic bioprosthesis dysfunction (usually due to aortic regurgitation) in keeping with the concept of one valve over another.12
Cardiac biological prostheses have a short mean life despite huge improvements made in the newer generations of prosthesis. In fact, at 15 years the percentage of patients with no aortic valve replacement was 58% in the case of first-generation Carpentier-Edwards prostheses (implanted in the late 1970s)4 and 83% in the case of second-generation prostheses (implanted in the 1980s and 1990s).13 Repeat procedures pose a very high risk to these patients because most are in their 80s. In the series described by Langenay et al.,14 which analyzed aortic valve surgery in octogenarians, the main predictor of mortality was reoperation. Therefore, a therapeutic approach that entails less risk may offer unquestionable advantages to more patients.
Another important issue is the age at implantation of a bioprosthesis versus mechanical prosthesis, as the controversy concerns the risk of reoperation compared to the risk of long-term anticoagulation. In a recent study, event-free survival would be higher after age 60 years in the case of the bioprosthesis.15 In the future, percutaneous implantation may be offered to high-risk patients instead of reoperation and, therefore, the use of mechanical prostheses plus anticoagulation therapy would be reserved for younger patients.
Because a percutaneous prosthesis would be implanted in these cases, a lower incidence of adverse phenomena (paravalvular leaks or cerebral strokes) could be expected, compared to the treatment of a calcified native aortic valve. We had no such complications in our patients; however, larger studies are needed to confirm this observation.
In conclusion, the outcomes obtained with percutaneous implantation of the CoreValve aortic prosthesis indicate that the treatment is feasible and achieves hemodynamic and clinical improvement in patients with aortic bioprosthesis dysfunction.
Conflicts of interestThe authors state that they have no conflicts of interest.
Received 27 January 2010
Accepted 9 March 2010
Corresponding author: Servicio de Cardiología, Hospital Universitario Virgen de la Victoria, Campus de Teatinos, s/n, 29010 Málaga, Spain. ajmunozgarcia@secardiologia.es