Despite ongoing development in stent design and the greater efficacy of antiplatelet therapy, stent thrombosis (ST) continues to be a widely recognized and much dreaded adverse event, with an incidence of 1% to 5%1,2 and a mortality that exceeds 10% in all series.3,4 In ST, the implantation of an additional stent has been related to an adverse outcome, higher risk of rethrombosis, and increased mortality.1,2 Intravascular ultrasound (IVUS) is an essential tool to identify the causal mechanism of ST; however, IVUS is rarely used to investigate ST in our setting.1,5
We describe IVUS findings obtained in definitive STs referred between 2008 and 2011 to our hospital and compare the therapeutic management of patients who underwent IVUS to that of patients not examined with IVUS.
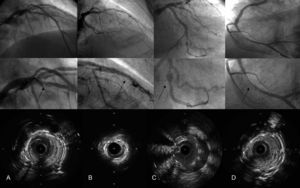
A total of 2028 patients with 3004 stents were treated and 45 definitive STs were reported, 18 (40%) of them investigated with IVUS (Table). IVUS was more likely to be used in acute and subacute ST than late or very late ST. In most cases, several ST-related mechanisms were identified: in patients with early ST (acute and subacute), underexpansion and lesion at the stent border were the most common echographic findings, whereas patients with late and very late thrombosis were most likely to show in-stent proliferation with severe stenosis and, in 1 case, malapposition due to positive vessel remodeling. The 4 ST mechanisms observed are shown in the Figure.
Intravascular Ultrasound Findings in Enrolled Patients and Therapeutic Management
| Patient | Age | Vessel | Stent | Time, d | Angiographic findings | Intravascular ultrasound findings | Therapeutic management |
| 1 | 68 | ADA | DES | 0 | Complete occlusion at proximal stent border | Underexpansion and adherent thrombus | Thrombus aspiration, abiximab, and postdilation |
| 2 | 70 | RCA | BMS | 1 | Complete occlusion at proximal stent border | Intimal flap at proximal stent border | Thrombus aspiration and implantation of new BMS |
| 3 | 69 | RCA | BMS | 4 | Image of organized stent thrombus, with TIMI 1 distal flow | Slight underexpansion. Severe lesion at distal stent border | Thrombus aspiration and implantation of new BMS |
| 4 | 72 | ADA | DES | 4 | Complete stent occlusion at overlap of tandem stents | Considerable underexpansion (360° calcium ring in vessel) (Figure B) | High-pressure postdilation with balloons of increasing size |
| 5 | 63 | ADA | DES | 4 | Complete occlusion at proximal stent border | Intimal flap at distal stent border, malapposition | Thrombus aspiration, abiximab, and implantation of a new DES |
| 6 | 69 | ADA | DES | 4 | Complete occlusion at proximal stent border | Stent underexpansion and malapposition | Thrombus aspiration, abiximab, and postdilation |
| 7 | 47 | RCA | DES | 4 | Complete stent occlusion (distal border) | Intimal flap at distal stent border with portal entrance distally visible but proximally trapped by stent; 60-90° arch | Thrombus aspiration, abiximab, and new DES |
| 8 | 76 | Cx | DES | 5 | Complete occlusion at proximal stent border | Severe underexpansion, malapposition | Thrombus aspiration, abiximab, and postdilation |
| 9 | 72 | RCA | BMS | 5 | V-shaped stents at bifurcation, with occlusion from stent origin in posterolateral artery | Underexpansion of posterolateral artery stent | Abciximab and postdilation |
| 10 | 40 | ADA | BMS | 8 | Complete occlusion anterior to stent with ruptured plaque image at proximal stent border | Intimal flap at proximal stent border. Adherent thrombus (Figure A) | Thrombus aspiration, abiximab, and new DES |
| 11 | 83 | Dx | BMS | 9 | Complete occlusion from stent origin in first Dx | Stent struts adjusted to Dx ostium, protruding into ADA; underexpansion | Thrombus aspiration toward ADA, abiximab, and postdilation |
| 12 | 64 | RCA | BMS | 14 | Stent thrombosis in middle RCA with TIMI 0 flow | Stent underexpansion | Thrombus aspiration, abiximab, and postdilation |
| 13 | 71 | GSV | DES | 240 | Critical focal in-stent restenosis at distal border of bridge (anastomosis toward OM) with TIMI 1 distal flow | Severe concentric hyperplasia | Thrombus aspiration, abiximab, and new DES |
| 14 | 58 | OM | DES | 290 | Image of stent thrombus with aneurysmal vessel dilatation at proximal stent border | Malapposition by positive vessel remodeling (Figure C) | Thrombus aspiration, abiximab, and postdilation; IVUS confirmation of outcome |
| 15 | 49 | RCA | DES | 350 | Complete occlusion at proximal stent border | Good stent apposition with no proliferation; adherent thrombus | Thrombus aspiration and abiximab |
| 16 | 69 | PIV | DES | 683 | Complete occlusion of stent (middle segment) implanted in distal RCA toward posterior descending artery | Underexpansion and adherent thrombus | Abciximab and postdilation |
| 17 | 70 | ADA | DES | 1858 | Image of organized stent thrombus with TIMI 1 distal flow | Stent underexpansion, malapposition, and residual thrombus | Thrombus aspiration, abiximab, and postdilation |
| 18 | 51 | RCA | BMS | 2221 | Complete occlusion of proximal border of first stent (2 overlapping stents) | Diffuse stent proliferation (probable neoatherosclerosis) with severe stenosis (Figure D) | Abciximab and DES implant |
| Therapeutic management in both groups | P | ||
| IVUS (n=18) | No IVUS (n=27) | ||
| Early thrombosis | 12 (66.7) | 5 (3.7) | .01* |
| Late thrombosis | 6 (33.3) | 14 (51.8) | .22 |
| Bare-metal stents | 7 (38.9) | 9 (33.3) | .71 |
| Drug-eluting stents | 11 (61.1) | 18 (66.7) | .71 |
| Aspiration | 14 (77.8) | 13 (48.2) | .05* |
| Abciximab | 15 (83.3) | 14 (51.8) | .03* |
| New stent implantation | 6 (33.3) | 17 (62.9) | .05* |
| Mortality | 4 (22.2) | 4 (14.8) | .52 |
| Rethrombosis | 1 (5.5) | 3 (11.1) | .52 |
ADA, anterior descending artery; BMS, bare-metal stent; Cx, circumflex artery; DES, drug eluting stent; Dx, diagonal artery; GSV, great saphenous vein; IVUS, intravascular ultrasound; OM, obtuse marginal artery; PIV, posterior interventricular artery; RCA, right coronary artery; TIMI: Thrombolysis in Myocardial Infarction.
Malapposition is defined as 1 or more stent struts separated from the vessel wall, except at the origin of a secondary branch. Underexpansion is defined as minimal stent area <80% of the mean proximal and distal reference area.
* Statistically significant results.
Four main mechanisms of stent thrombosis. The upper row shows the coronary angiographies once distal flow is recovered, the middle row provides a zoomed view of the stent, and the bottom row contains intravascular ultrasound cross-sectional images. A: Intimal flap at the stent border. B: Stent underexpansion. C: Malapposition due to positive vessel remodeling. D: Neointimal proliferation with severe stenosis.
In terms of therapeutic management, patients with late thrombosis most often required balloon predilation to advance the IVUS probe, which could overestimate the minimum stent area. In 17 patients, IVUS identified the definitive cause of thrombosis. The symptoms were related to discontinuation of dual antiplatelet therapy in only 1 patient with late thrombosis, and IVUS study revealed no pathologic findings. The use of glycoprotein IIb-IIIa inhibitors and thrombosis aspiration devices was more common in the group of patients assessed with IVUS. STs examined by IVUS were treated less often with implantation of a second stent. In fact, IVUS study made it possible to orient and optimize treatment in all patients. No significant differences were detected in angiographic outcome, mortality, or rethrombosis.
The IVUS findings of early and late ST in our series presented different profiles, which could indicate that these entities have different pathophysiologic mechanisms. The relationship between early thrombosis and mechanical factors during the implant procedure has already been reported in previous studies. Cheneau et al.6 found that subacute ST and inadequate outcome in the implantation procedure was related to significantly smaller stent areas and other echographic findings, such as dissection, residual thrombus, or tissue prolapse between the struts. In the largest published register, Amstrong et al.4 identified multiple clinical, angiographic, and prognostic factors based on the point in time of the STs, which would indicate that each entity must correspond to a different etiologic mechanism. Additionally, these authors observed a stronger tendency toward stent implantation in very late thrombosis than in early thrombosis.
Implantation of an additional stent in thrombosis conditions was identified as an independent predictive factor of mortality and ST recurrence in the ESTROFA register.1 In our series, patients who underwent IVUS were less likely to receive a second stent as part of thrombosis management, but no differences in mortality or rethrombosis were observed. However, IVUS was more often used in early than late thrombosis, which could overestimate the value of IVUS in preventing implantation of a second stent.
IVUS is highly useful for investigating the causal mechanism of thrombosis symptoms, as it reveals pathophysiologic factors underestimated by conventional angiography and identifies patients who may benefit from implantation of an additional stent.
.