The cardiac remodeling and left ventricular fibrosis that occur after myocardial infarction (MI) are important mediators of later development of heart failure leading to cardiovascular death.1
Following MI, in addition to repair fibrosis, there is a remodeling of the extracellular matrix and an increase in collagen synthesis that affects the myocardium at sites far from the affected necrotic portion.2 The relationship between myocardial strain, evaluated by speckle-tracking echocardiography, and the fibrosis that takes place after MI is unknown. This study analyzes the utility of radial strain (RS) and circumferential strain (CS) to assess the degree of repair fibrosis and remote fibrous tissue at the left ventricular in an experimental MI rat model.
Sham surgery was performed on 6 Wistar rats, and 6 others underwent ligature of the left anterior descending artery. Six weeks later, the rats were sacrificed.
All animals underwent echocardiography before and at one month after the procedure to obtain long-axis and short-axis images at the level of the papillary muscles. The left ventricular ejection fraction was calculated by both the Teichholz method and the Simpson method. The EchoPAC™ v110.1.2 software (GE Healthcare; Waukesha, Wisconsin, United States) was used for the analysis of short-axis images with speckle-tracking.
Hearts were sectioned at the papillary muscles, 6-µm sections were prepared and stained with Masson trichrome, and the size of the infarct was measured every 60 µm. Using the values obtained, the size of the MI was calculated in each heart and expressed as the percentage of infarcted area relative to the total myocardial area.
Collagen III concentration was determined by Western blot in the basal sections.
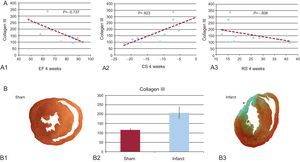
In all MI cases, the infarcted area showed histological features consistent with fibrosis (Figure). In parallel, Western blot study of areas at a distance from the infarcted tissue showed significantly higher collagen III expression in rats with MI than in sham-operated rats (Table and Figure).
A: correlation between collagen III and the ejection fraction (A1), circumferential strain (A2), and radial strain (A3). B1 and B3: histological section at the papillary muscles obtained from a sham-operated rat (B1) and a rat with myocardial infarction (B3). B2: concentration of collagen III in an area far from the infarcted tissue (sham vs myocardial infarction). CS, circumferential strain; EF, ejection fraction; RS, radial strain. *Anterior segment transmural scar.
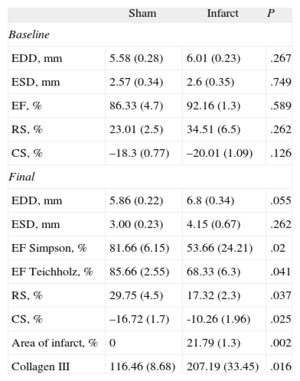
Western Blot Results for Areas Distant From the Infarcted Tissue in Rats With Myocardial Infarction vs Sham-operated Rats
| Sham | Infarct | P | |
| Baseline | |||
| EDD, mm | 5.58 (0.28) | 6.01 (0.23) | .267 |
| ESD, mm | 2.57 (0.34) | 2.6 (0.35) | .749 |
| EF, % | 86.33 (4.7) | 92.16 (1.3) | .589 |
| RS, % | 23.01 (2.5) | 34.51 (6.5) | .262 |
| CS, % | –18.3 (0.77) | –20.01 (1.09) | .126 |
| Final | |||
| EDD, mm | 5.86 (0.22) | 6.8 (0.34) | .055 |
| ESD, mm | 3.00 (0.23) | 4.15 (0.67) | .262 |
| EF Simpson, % | 81.66 (6.15) | 53.66 (24.21) | .02 |
| EF Teichholz, % | 85.66 (2.55) | 68.33 (6.3) | .041 |
| RS, % | 29.75 (4.5) | 17.32 (2.3) | .037 |
| CS, % | –16.72 (1.7) | -10.26 (1.96) | .025 |
| Area of infarct, % | 0 | 21.79 (1.3) | .002 |
| Collagen III | 116.46 (8.68) | 207.19 (33.45) | .016 |
CS, circumferential strain; EDD, end-diastolic diameter; EF, ejection fraction; ESD, end-systolic diameter; RS, radial strain.
At baseline, both groups presented similar RS and CS values at the papillary muscles (Table). However, at 4 weeks after the intervention, rats that underwent ligature of the anterior descending artery presented significantly lower overall RS and CS values then the sham-operated group (Table).
At 4 weeks after the procedure, CS values were significantly correlated with the end-diastolic diameter (?=0.59; P=.04) and the end-systolic diameter (?=0.788; P=.002). For RS, negative correlations were obtained for both the end-diastolic diameter (?=–0.56; P=.055) and the end-systolic diameter (?=–0.55; P=.059), with a trend to statistical significance that would likely be confirmed as significant in a larger sample size.
A significant correlation was observed between the size of the MI and collagen III concentrations (?=0.709; P=.01). We also found a significant correlation between the size of the MI and ejection fraction estimated by the Simpson method, RS, and CS (?=–0.783, ?=–0.709, and ?=0.729, respectively; P<.01 in all).
Collagen III concentrations correlated negatively with the ejection fraction, RS, and CS (?=–0.737, ?=–0.608, and ?=0.823, respectively; P<.05 in all) (Fig.).
Following MI, together with repair fibrosis, an increase occurs in extracellular matrix replacement and collagen synthesis that affects the non-necrotic segments and originates remodeling.2 Although several studies have shown the utility of strain as a predictor of adverse remodeling following MI,3 the relationship between the reduction in strain values and the fibrosis phenomena occurring after MI remain uncertain.
In keeping with the results of previous studies,4,5 we found a significant reduction in RS and CS values in our rat MI model and a correlation between the size of the infarction scar and the reduction in strain. Furthermore, we documented greater type III collagen expression in myocardial areas far from the infarcted region that correlated with RS and CS values at the papillary muscles. This correlation would indicate that the regional RS and CS are not only a reflection of the status of myocardial function affected by the scar, but also the increase in collagen synthesis that takes place in the extracellular remodeling process occurring after MI.6 The correlation observed between CS and collagen III was slightly stronger than that seen between RS and collagen III. This may be because RS is a function derived from the longitudinal and circumferential shortening, whereas CS is the result of direct measurement of the circumferential shortening in a specific region of myocardium.
The main limitation of this study is that the measurements of strain and infarcted area were taken in only one plane (short axis view at the papillary muscles) due to the difficulty of obtaining views in different planes in rats.
In conclusion, this study documents a correlation of the size of the infarction scar and remote fibrosis with the RS and CS. Strain could be a marker of myocardial fibrosis following MI.
FUNDINGThis study was partially supported by a research grant from Spanish Society of Cardiology.
.