Studies in other countries have reported an unfavorable prognosis in patients prescribed an inappropriate dose of direct oral anticoagulant (DOAC)1,2; however, corresponding information for Spain is lacking. The factors associated with the prescription of inappropriate DOAC doses were analyzed in a previous study of the FANTASIIA registry, a large Spanish registry of atrial fibrillation patients in routine clinical practice.3 The aim of the present study was to investigate the prognostic impact of inappropriate DOAC doses in this registry.
The FANTASIIA registry4 included atrial fibrillation outpatients on anticoagulant therapy (excluding patients younger than 18 years and those with a cardiac prosthesis, any degree of mitral stenosis, or moderate or severe mitral regurgitation). Patients were recruited consecutively at 50 Spanish centers between June 1, 2013 and October 15, 2014. The goal of the registry was to assess the effectiveness of anticoagulation therapy according to the type and quality of treatment. Of the total population, 530 patients received DOAC therapy, and these patients formed the study population for the present study. Patients were classified into dose groups according to whether the dose they received was appropriate, inappropriately high, or inappropriately low. The appropriateness of a low DOAC dose was defined according to the 2015 European Heart Rhythm Association clinical practice guidelines5 (table 1). The outcome measures were the associations between the prescribed dose and embolic events (stroke and systemic embolism), major bleeding, and mortality.
Criteria used in this study to define a low direct oral anticoagulant dose as appropriate
| For patients receiving dabigatran: |
| 1. Age ≥ 80 y |
| 2. Use of verapamil |
| 3. ≥ 2 of the following: age 75-79 y; creatinine clearance = 30-50 mL/min; HAS-BLED ≥ 3; use of amiodarone or antiplatelet drugs; body weight ≤ 60 kg |
| For patients receiving rivaroxaban: |
| 1. Creatinine clearance = 15-49 mL/min |
| 2. ≥ 2 of the following: age 75-79 y; creatinine clearance = 30-50 mL/min; HAS-BLED ≥ 3; use of amiodarone or antiplatelet drugs; body weight ≤ 60 kg |
| For patients receiving apixaban: |
| 1. ≥ 2 of the following: age> 80 y; creatinine ≥ 1.5 mg/dL; body weight ≤ 60 kg |
| 2. Creatinine clearance = 15-29 mL/mn |
| 3. ≥ 2 of the following: age ≥ 75 y; HAS-BLED ≥ 3; use of amiodorone, diltiazem, or antiplatelet drugs |
HAS-BLED: hypertension, abnormal renal or hepatic function (1 point for each), a history of stroke, a history of or predisposition to bleeding, labile international normalized ratio, age > 65 years, concomitant use of alcohol or drugs (1 point for each).
In the study sample, the prescribed dose was appropriate in 358 patients (67%), inappropriately low in 93 (18%), and inappropriately high in 79 (15%). Baseline patient characteristics differed significantly between the dose groups.3
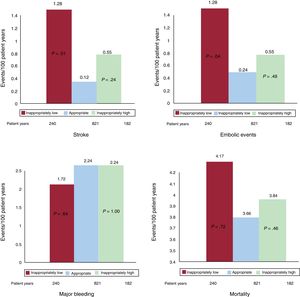
During a maximum 3-year follow-up (1243 patient years), there were 6 embolic events (5 strokes and 1 systemic embolism), 26 major bleeding events, and 47 deaths. The unadjusted event rates by dose group are shown in figure 1. On multivariate comparison with an appropriate dose, an inappropriately low dose was associated with a higher risk of stroke (hazard ratio [HR]=16.7; 95% confidence interval [95%CI], 1.7-164.4; P=.016) and all embolic events (HR=7.3; 95%CI, 1.2-44.5; P=.03). There were no between-group differences in major bleeding (HR=0.79; 95%CI, 0.27-2.33; P=.67) or mortality (HR=2.13; 95%CI, 0.93-4.9; P=.07). In comparison with an appropriate dose, an inappropriately high dose was not associated with any of the events (stroke: HR=3.3; 95%CI, 0.2-53.0; P=.4; embolic events: HR=1.8; 95%CI, 0.2-19.7; P=.64; major bleeding: HR=0.81; 95%CI, 0.27-2.42; P=.7; death: HR=0.88; 95%CI, 0.37-206; P=.76).
A link between inappropriate DOAC dose and poor prognosis was reported in earlier studies. A retrospective analysis of an administrative database reported high rates of stroke with inappropriately low doses of apixaban and an increased risk of bleeding with inappropriately high DOAC doses.1 A prospective multicenter registry2 showed an increase in cardiovascular hospital admissions among patients receiving an inappropriately low DOAC dose and an increase in mortality linked to inappropriately high doses. Both of these studies were carried out in the United States, where the health system and prescription patterns differ from those in Spain. The present study confirms a similar pattern in Spain. The limitations of our study include the low event rate, the small sample size (which prevented analysis of the results by type of DOAC), and the absence of patients receiving edoxaban. Moreover, dose appropriateness in our study was defined according to the 2015 European Heart Rhythm Association criteria; these tended to be lenient on the use of doses below those stipulated in the medication package insert, and this has been corrected in the 2018 update.6 Finally, our registry data lacked adequate information on the persistence, interruption, suspension, or modification of anticoagulant therapy; the stability of renal function; and other circumstances arising during the study that might have influenced the occurrence of events, thus limiting the multivariate adjustment to data collected at the baseline visit. Nevertheless, our results confirm that, in our setting and in a varied sample of atrial fibrillation patients treated in routine clinical practice, the prescription of inappropriately low DOAC doses is associated with a significantly increased risk of embolic events.
FUNDINGThis study was supported by a Pfizer/Bristol-Myers-Squibb Research Grant.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.09.014