Pulmonary arterial hypertension (PAH) occurring after correction of D-transposition of the great arteries (D-TGA) is a condition still under investigation. The pioneering technique for treating D-TGA was atrial switch (Mustard or Senning procedure) performed in the first 5 to 8 months of life. The reported incidence of PAH after this type of repair was 7%, attributed to cyanosis, endothelial injury, hyperoxia of the pulmonary arterial tree, and persistence of pulmonary-systemic shunts up to the time of surgery. Later, this treatment was replaced by anatomical correction or arterial switch performed in the first days of life. However, PAH still develops in some patients after arterial switch. Hence, other mechanisms have been proposed to explain this event: abnormal development of the pulmonary vascular tree during fetal growth (early closure of the fetal foramen ovale or pulmonary hypoxia), pulmonary embolic events during Rashkind atrial septostomy, and even certain genetic factors.1
Our aim was to analyze the prevalence of PAH-related genetic variants in a cohort of patients with repaired D-TGA and PAH, recorded in the REHIPED registry (Spanish Registry of Pediatric Pulmonary Arterial Hypertension).2 Patients were diagnosed by right cardiac catheterization, with the exception of clinically unstable cases. Genetic analysis was caried out using an NGS panel of 21 genes: ABCC8, ACVRL1, BMPR1B, BMPR2, CAV1, CBLN2, CPS1, EIF2AK4, ENG, GDF2, KCNA5, KCNK3, MMACHC, NOTCH3, SARS2, SMAD1, SMAD4, SMAD5, SMAD9, TBX4, and TOPBP1.3 Variants were classified according to recommendations of the American College of Medical Genetics. Written informed consent was obtained from parents or legal guardians in all cases. The REHIPED registry was approved by the Medical Research Ethics Committees of the participating centers.
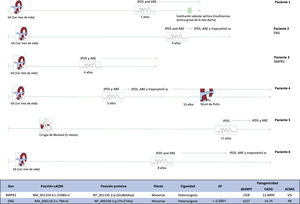
Between 2011 and 2020, 9 children with repaired D-TGA and PAH were candidates to participate, and 6 agreed to undergo a genetic study (4 male and 2 female individuals) were included. Five patients had undergone arterial switch and 1 (patient 5) had received Mustard surgery. Three participants (patients 2, 3, and 5) had a complete interventricular septum. Patient 1 was diagnosed at 7 years of age (mean pulmonary arterial pressure [mPAP], 34mmHg; pulmonary vascular resistance index [PVRi], 4.3 UW/m2) and has remained in functional class (FC) II with dual oral therapy. Although this patient had severe aortic regurgitation requiring aortic valve replacement at the age of 10 years, pulmonary capillary pressure (PCP) was found to be <15mmHg in successive catheterizations performed before surgery and at 1 year after. Patient 2 was diagnosed at 9 years of age (PAPm, 65mmHg; PCP, 14mmHg; PVRi, 30 UW/m2), and is stable at 14 years with triple therapy that includes subcutaneous treprostinil. Patient 3 was diagnosed at 4 years of age (highly probable on transthoracic echocardiography findings, and right heart catheterization impossible because of clinical instability). She was started on dual oral therapy and 3 years later remains in FC II; on follow-up, right catheterization has been excluded due to a persistent, general low-risk situation. Patient 4 was diagnosed at 5 years of age (PAPm, 63mmHg; PCP, 9mmHg; PVRi, 17.4 UW/m2). Despite triple therapy that included treprostinil, the patient required a Potts shunt at age 11 years. She is now in FC II at 21 years of age. In patient 5 (Mustard surgery at 5 months), PAH was confirmed at the age of 17 years (PAPm, 62mmHg; PCP, 7mmHg; PVRi, 12 UW/m2). The patient is now 30 years old and remains in FC II with dual oral treatment. Patient 6 was 6 years old at the diagnosis (PAPm, 85mmHg; PCP, 10mmHg; RVPi, 17 UW/m2) and remains in FC II with triple therapy including subcutaneous treprostinil (figure 1).
In silico analysis of candidate genes. The hg19 genome was used as a reference. The prediction used aggregated databases in dbSNFP, CADD, and ACMG. Allelic frequency was estimated based on multiple pseudo-control populations: gnomAD genomes (v2.1.1), gnomAD exomes (v2.1.1), Kaviar (version 160204-Public), Beacon (v2.0), 1000G, Phase III, and Bravo. AF, allelic frequency; ERA, endothelin receptor antagonists; PDE5i, phosphodiesterase 5 inhibitors; PB, probably benign; AS, arterial switch; VUS, variant of uncertain significance.
Genetic study identified a probably benign variant in the ENG (endoglin) gene and a variant of uncertain significance in BMPR2. Although in silico analysis initially classified the ENG variant as having uncertain significance, presence of the varient in the patient's asymptomatic father led to its reclassification as probably benign. In patient 3, in silico analysis initially classified the variant as probably pathogenic, but it was reclassified as having uncertain significance after it was confirmed in the patient's father, who was also asymptomatic.
This is the first study investigating the possible influence of PAH-related genetic variants on the development of this condition following D-TGA correction. Although the variants found are not considered pathologically significant, their potential role cannot be excluded in the presence of D-TGA. Because of the small sample size and analysis limited to currently known PAH-related genes, the genetic hypothesis in the development of PAH cannot be ruled out. A recent genome-wide association study (GWAS) in a cohort of 133 patients with D-TGA found a relationship between certain genetic polymorphisms and an improvement in the prognosis following arterial switch.4 New studies are needed to bring to light possible genetic factors involved in the development of PAH in D-TGA patients. It may be necessary to use techniques such as GWAS to search for new implicated genomic regions.4
FUNDINGA. Cruz-Utrilla received financial support as part of the Río Hortega grant (CM20/00164) from the Spanish Ministry of Science and Innovation (Carlos III Health Institute). This study was cofinanced by funds from the Health Research Fund of the Carlos III Health Institute (FISPI18/01233).
AUTHORS’ CONTRIBUTIONSAll authors have contributed to the design, writing, and critical analysis of the article.
CONFLICTS OF INTERESTThe authors have no conflicts of interest related to this article.
.