Surgically implanted prosthesis dysfunction is one of the clinical situations in which percutaneous implantation of a new prosthesis (procedure known as valve-in-valve) has produced good outcomes.1 One of the major concerns in these cases is the risk of coronary obstruction, which has an incidence of around 2.3% to 3.5%.1,2 The development of coronary obstruction is related to displacement of the leaflets of the malfunctioning bioprosthesis toward the coronary ostium following expansion of the percutaneous valve.2 This complication is associated with high mortality and can occur immediately after the procedure or within days (in the VIVID registry, 36.1% of obstructions were delayed).2,3 There are reports of chimney stent implantation in patients who experience coronary obstruction during prosthesis release.4,5 However, no publications describe the use of this type of procedure for pre-emptive left main stenting to avoid late obstruction. We report a case with a step-by-step description of this technique.
A 79-year-old man with a history of inferior infarction with papillary muscle rupture in 2003 underwent surgical implantation of a 27-mm Sorin Bicarbon mechanical prosthesis and saphenous vein graft to the anterior interventricular artery. In 2010, a 21-mm Mitroflow biological prosthesis was implanted due to severe degenerative aortic stenosis.
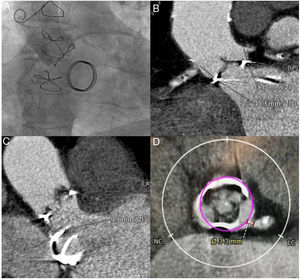
In September 2018, the patient was admitted for heart failure secondary to aortic bioprosthesis dysfunction, with severe intraprosthetic aortic regurgitation. Echocardiography also showed severe left ventricular dysfunction, with inferoposterior necrosis, and a normal-functioning mitral mechanical prosthesis. The patient had no clinical, laboratory, echocardiographic, or microbiologic evidence of infective endocarditis, which was definitively ruled out by positron-emission tomography. Coronary angiography showed severe 3-vessel coronary disease with a patent saphenous vein bypass to the anterior interventricular artery (figure 1AA). To evaluate the valve complex and vascular accesses, computed tomography angiography was performed, detecting as the greatest limitation for percutaneous aortic valve implant a low height of the left main coronary artery (LMCA) (height of 8.6mm above the prosthetic ring) (figure 1B) and narrow sinuses of Valsalva. The distance from the aortic prosthetic ring to the mitral prosthesis was 9.9mm (figure 1C). In the simulated reconstruction, the distance at the sinuses of Valsalva between the virtual transcatheter ring and the origin of the LMCA was 3.3mm (figure 1D). A distance <4mm has been associated with a high risk of coronary obstruction.2
A: aortogram showing low left main height and narrow sinuses of Valsalva. B: computed tomography angiography measurement of the distance between the bioprosthetic ring and the 2 coronaries. C: computed tomography angiography measurement of the distance between the aortic biological prosthesis and the mitral mechanical prosthesis. D: calculation of the distance between the virtual ring of the 23-mm transcatheter prosthesis and the origin of the left main coronary artery. LC, left coronary; NC: noncoronary.
The patient was readmitted in December 2018 due to a new episode of refractory heart failure. The case was discussed in the medical-surgical session and, in view of the high surgical risk (logistic EuroSCORE of 47.2% and Society of Thoracic Surgeons score of 11.7%), a decision was made to implant an Evolut RTM valve (Medtronic; Minneapolis, Minnesota, United States) of 23mm.
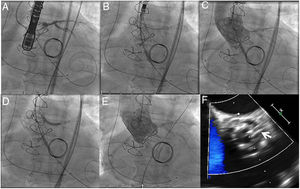
The procedure via femoral access was planned for pre-emptive chimney stenting in the LMCA due to the high risk of coronary obstruction, in light of the external position of the Mitroflow valve leaflets, the measurements reported, and recent reports that a third of all obstructions are delayed.2 The procedure was performed under general anesthesia and transesophageal ultrasound guidance. Double femoral and double radial access was used to protect both coronaries. A BHW angioplasty guidewire was placed in both coronaries (figure 2A), and a drug-eluting stent of 3.5 x 26mm was positioned in the LMCA, causing half of the artery to prolapse into the aortic root to completely reject the bioprosthetic leaflets (figure 2B). Once transesophageal ultrasound confirmed the absence of interference with the mitral prosthesis and the patency of the coronary vessel, the 23-mm Evolut R prosthesis was released with no incidents (figure 2C). Following release, the stent previously positioned in the LMCA was implanted to reject the Mitroflow prosthesis leaflet and to reduce the risk of late coronary obstruction (figure 2D). Imaging confirmed the absence of aortic regurgitation and the patency of both coronaries (figure 2E, 2F). The patient was discharged 8 days later. At the 2-month clinical follow-up visit, he was asymptomatic, with follow-up ultrasound showing a normally functioning aortic prosthesis and a mean gradient of 24mmHg, with no regurgitation.
A: positioning of super-stiff guide wire in left ventricle and 2 angioplasty guide wires in the right coronary and anterior interventricular arteries. B: partial deployment of the percutaneous valve; the image shows the stent previously positioned in the left main coronary artery; half of the artery prolapses into the ascending aorta. C: released percutaneous prosthesis. D: stent implantation in the coronary artery (chimney stenting). E: final follow-up angiogram showing no aortic regurgitation and patency of both coronaries. F: transesophageal ultrasound imaging of the chimney stent (arrow), showing the metal mesh of the stent outside the transcatheter prosthesis ring.
The risk of coronary obstruction is determined by the characteristics of the surgical bioprosthesis (more common in stentless bioprostheses and stented bioprostheses with externally mounted leaflets, as in the Mitroflow), its position in relation to the coronary origin, and the anatomy of the aortic root. Pre-emptive left main stenting in valve-in-valve procedures can be safely performed to reduce the risk of both early and late coronary obstruction caused by bioprosthetic leaflet displacement toward the coronary ostium. Chimney stenting does not avoid the potential risk of in-stent thrombosis (which requires careful monitoring of antiplatelets) and may hinder the possibility of subsequent surgeries on the left coronary vasculature.