Keywords
INTRODUCTION
Atrial septal aneurysms are localized, bulging malformations that protrude into the right or left atrium. This malformation can be associated with patent foramen ovale (PFO), atrial septal defects (ASD) or multiple defects, and is considered a risk factor for stroke.1-4 Growing experience with new occlusion devices5,6 has allowed these patients to undergo percutaneous treatment. In this study, we analyze results of treatment of this malformation with Amplatzer occluder devices.
METHOD
Patients
Between June 1993 and February 2004, 203 patients with ASD or PFO underwent percutaneous closure; 29 (14%) had atrial septal aneurysm. In the transesophageal study, all the adults met the following criteria1: a) base diameter >15 mm, and b) excursion 15 mm beyond the interatrial septum plane. Six children of 5-12 years presented the same morphology but on a smaller scale (aneurysm base 8 mm/m2 body surface area).7
Diagnostic Catheterization
The procedure was performed under general anesthesia and transesophageal monitoring. Following angiographic and echocardiographic measurements, 1 mg/kg of intravenous heparin was administered and measurements were completed by the stretching balloon method. Patients with PFO were injected with 5 ml of shaken saline solution in the inferior cava to show presence of right to left shunt (Figure 1) and this was repeated during an intermittent positive pressure maneuver performed by the anesthetist.
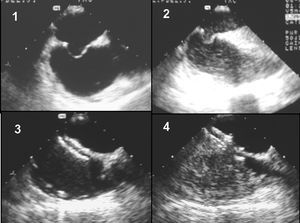
Figure 1. Patient with patent foramen ovale associated with aneurysm: 1) baseline transesophageal study; 2) following injection of physiological saline solution in the cava, a significant contrast shunt in the left atrium is seen; 3) Amplatzer device occludes the defect; 4) absence of contrast shunt following implantation.
Therapeutic Phase
We used Amplatzer occluders (Golden Valley, Minnesota) and varied implantation strategies according to anatomy. In patients with a single orifice, just 1 occluder was implanted. The device chosen was of a diameter suitable for deployment by balloon occlusion. Patients with multiple orifices received 1, 2, or 3 devices according to the site of the malformation (foramen ovale or entire septum) and proportion of thin septum compared with the thicker remains of the real septum. Patients with a small hole near the major orifice also received a single occluder. Five patients with separate orifices received 2 or 3 devices. In these procedures we used a simultaneous double balloon occlusion technique to measure the exact size of the defects (Figure 2). Following the procedure, all patients received subcutaneous dalteparin for 1 month and aspirin for 6 months. Patients with a history of stroke were assigned an antiplatelet regimen indefinitely. We defined primary success as echocardiographic absence of residual shunt at discharge. Noninvasive clinical follow-up was carried out at 3, 6, and 12 months post-treatment and yearly thereafter.
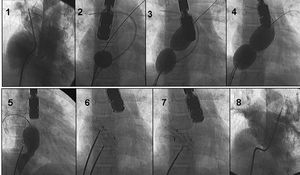
Figure 2. Patient with 2 separate orifices who receives 2 occluders: 1) baseline angiography in superior right pulmonary vein; 2-4) measuring defect size with occlusion balloon; 5) after implantation of a first device the residual defect is measured; 6-7) implantation of a second device; 8) occlusion of all defects as shown in pulmonary arteriography.
We performed paired Student-Fisher test to compare each patient's mean values.
RESULTS
Baseline and procedure data are summarized in Table 1. In order to close the entire aneurysm, the 10 patients with PFO received 35 mm Amplatzer PFO occluders. In patients with ASD, we found a significant reduction of pulmonary pressure and pulmonary/systemic flow ratio (Table 2). We often found mild residual shunting in the post-immediate study (Table 2). However, this disappeared in the transthoracic echocardiographic evaluation performed prior to discharge in all but 1 patient (Table 2). In patients with PFO, transesophageal echocardiography performed in the laboratory showed a significant right-left shunt in baseline conditions that became massive following the positive pressure ventilation maneuver (Table 3). Immediately after implantation, all these patients presented without shunt in baseline conditions (Figure 1) whereas during the positive pressure maneuver we identified a small <10-15 microbubble right-left shunt in the left atrium in 6 patients (Table 3).
Complete closure was found in all patients after a mean follow-up of 31±19 months. In 1 patient with residual shunt at discharge, echo-Doppler at 6 months showed complete closure (Table 2). In patients with PFO, transthoracic echocardiography at 6 months showed an absence of residual shunt after intravenous injection of saline solution in the antecubital vein and Valsalva maneuver (Table 3). Patients with dyspnea at the time of the procedure presented improved functional status. No embolic events occurred during follow-up.
DISCUSSION
Atrial septal aneurysms are infrequent malformations which have begun to be understand only recently following systematic use of 2D echography.8
Atrial Septal Aneurysm Associated With Patent Foramen Ovale
The mechanisms implied in embolic episodes in patients with atrial septal aneurysm are related with the right-left passage through the PFO, the presence of supraventricular arrhythmias or the formation of thrombus in the aneurysm itself.2-4 Patients with a first ischemic embolism have a 15% risk of a second event in a 4 years period even when they receive aspirin.4 Mechanical closure of the defect seems a logical alternative therapy (Figure 1).
Table 4 summarizes the principle series published.7,9-13
Comparing results of these studies is difficult given the differences in the devices used (7 types). Most publications describe a high percentage of success with a very low incidence of embolic events during follow-up. In fact, implanting one of these devices in patients with PFO, whether associated with atrial septal aneurysm or not, is relatively easy. However, the unanswered questions focus on the long-term efficacy of these procedures and the appropriate selection of candidates for PFO closure.
Atrial Septal Aneurysm Associated With Interatrial Defect
Experience of percutaneous closure this type of association is more limited than in patients with PFO (Table 4). Given that multiperforated septal defects are frequent (Figure 3), this increases the complexity of the percutaneous intervention.5 Consequently, surgery is preferred in many centers. When the decision is taken to perform a percutaneous procedure, the implantation strategy must be carefully considered. The simplest strategy is to implant a single device that traps the entire aneurysm between the disks. However, sometimes more than 1 device is needed and different measurements of the defect are required in order to make the final decision (Figures 2 and 3). Given the discrepancies in defect measurement between echography and balloon, we believe procedures should not be undertaken without previously using the stretching balloon method.
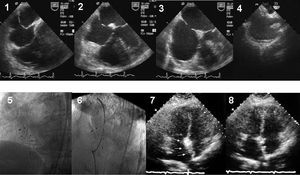
Figure 3. Patient with multiperforated atrial septal aneurysm (1-3). Following implantation of a first device (4), 2 additional devices are required (5 and 6). At 6 months, transthoracic echocardiography shows reconstruction of a thicker interatrial septum without residual shunt (7 and 8).
Our study suggests that patients with an atrial septal aneurysm associated with an ASD can be treated successfully by implanting an Amplatzer occluder. However, patients with multiperforated septum (Figure 3) should be intervened by teams of experts.
Correspondence: Dr. M. Pan.
Grupo CORPAL. Hospital de la Cruz Roja.
Paseo de la Victoria, s/n. 114 Córdoba. España.
E-mail: grupo_corpal@arrakis.es