Coronary fistulas are uncommon congenital or acquired anomalies that have a low incidence in the general population (0.3%-0.8%). Most of them originate in right coronary artery (50%-60%) or anterior descending artery (30%-40%); the circumflex artery (Cx) is the vessel least often affected.1 Closure of symptomatic fistulas is indicated from the time of their diagnosis or when associated with myocardial ischemia, arrhythmias, or ventricular dilation or dysfunction, regardless of their size. The treatment in asymptomatic patients is controversial, and some authors recommend closure because of the risk of late complications.1,2 The detection of these anomalies in adults is associated with higher surgical risk and an increased rate of comorbidities. For decades, surgery was the standard treatment, and transcatheter closure was performed only in patients in whom surgery was not an option and/or those having a favorable anatomy. Nevertheless, percutaneous treatment is feasible in experienced centers. We report 2 cases of transcatheter closure of fistulas in the circumflex artery in adult patients with major comorbidities and complex vascular anatomy, who had refused to undergo surgery.
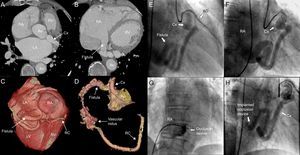
The first patient was a 77-year-old man with hypertension and permanent atrial fibrillation who was receiving oral anticoagulation therapy. He was referred to us because of dyspnea on mild exertion. Transthoracic echocardiography revealed marked dilatation of right chambers and severe tricuspid regurgitation. Cardiac catheterization showed a normal pulmonary artery pressure and left-to-right shunt with a pulmonary flow to systemic flow ratio (Qp/Qs) of 1.7. Coronary angiography revealed a tortuous, dilated Cx that drained into right atrium, a finding that was corroborated by computed tomography (Figures 1A-D).
Computed tomography (A and B) with 3-dimensional reconstruction (C and D), showing a dilated circumflex artery draining into a highly dilated right atrium, its anatomical position, and its distal connection to right coronary artery via a tangle of vessels. Angiographic images of the fistula between circumflex artery and right atrium (E), the arteriovenous loop (F), and the delivery of the occlusion device (G), which resulted in the absence of significant residual flow (H). AD, anterior descending artery; Ao, aorta; Cx, circumflex artery; LA, left atrium; LV, left ventricle; RA, right atrium; RC, right coronary artery; RV, right ventricle.
Vascular access for transcatheter closure was gained via right femoral artery and left femoral vein (6-Fr catheters were employed for both approaches). Using an Amplatz left catheter, the fistula was cannulated from left main coronary artery, and an extra-flexible guide wire was advanced from the arterial side to its entrance into right atrium. To establish the arteriovenous loop, we used a lasso catheter to snare the coronary guide wire in right atrium, and exteriorize it through left femoral vein. The fistula was then cannulated via left femoral vein with a multipurpose catheter, by way of which a 10-mm Amplatzer® Vascular Plug (AVP) (AGA; Minnesota, United States) was successfully implanted where the fistula was narrowest. Coronary angiography showed a minimal residual shunt, with adequate device positioning and stability (Figures 1E-H).
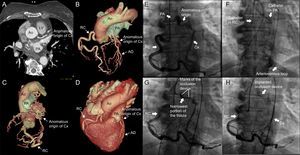
The second patient was a 68-year-old woman with diabetes, hypertension, and grade II obesity, who was receiving oral anticoagulation therapy. She was admitted to the hospital with chest pain and de novo atrial fibrillation. Coronary angiography revealed no significant coronary lesions, but did show an anomalous origin of Cx arising from pulmonary artery. Anterior descending artery had a normal origin. Right heart catheterization disclosed a Qp/Qs of 2.5, with normal pulmonary artery pressure. Computed tomography confirmed the tortuosity and ectasia of the Cx, which arose from proximal right pulmonary artery, ran along the atrioventricular groove, gave rise to an obtuse marginal branch, and received collateral vessels from the bronchial arteries, ending in a posterolateral branch anastomosed directly to posterior descending artery, which originated from the dilated, tortuous right coronary artery (Figures 2A-D).
Computed tomography (A) with 3-dimensional reconstruction (B-D) showing the dilated circumflex artery, with an anomalous origin in right pulmonary artery, its course, and the distal anastomosis to right coronary artery by way of collateral branches. Angiographic images demonstrating the anomalous origin of the circumflex artery in pulmonary artery, its distal anastomosis to posterior descending artery (E), the arteriovenous loop (F), the positioning of the device (G), and its successful delivery, which achieved complete occlusion of the fistula (H). AD, anterior descending artery; Ao, aorta; Cx, circumflex artery; LV, left ventricle; PA, pulmonary artery; RC, right coronary artery.
For percutaneous closure, vascular access was gained via right femoral artery and left femoral vein by means of 6-Fr catheters. Right coronary artery was cannulated using a Judkins right catheter; with the aid of a microcatheter, an extra-flexible guide wire was advanced in the anterograde direction, along a collateral epicardial branch that originated in right coronary artery, to reach the origin of the Cx in right pulmonary artery. The arteriovenous loop was established by snaring the guide wire with a lasso catheter and exteriorizing it via left femoral vein. An AVP II device was immediately implanted, by way of the venous access to the pulmonary artery, to successfully occlude the fistula (Figures 2E-H).
Both patients were discharged from the hospital with chronic oral anticoagulation therapy. One year after the intervention, they remained asymptomatic and free of complications.
Individuals with fistulas can remain asymptomatic for years. When symptoms appear, they are mainly a consequence of cardiac ischemia resulting from coronary steal, heart failure produced by volume overload due to the left-to-right shunt, infective endocarditis, arrhythmias, endarteritis, and fistula rupture or thrombosis. The guidelines for the treatment of congenital heart disease in adults recommend the closure of all large or hemodynamically significant fistulas, regardless of the symptoms, using surgical or percutaneous techniques.3 Before the advent of percutaneous management, surgery was the most common approach. The main criteria influencing the choice of treatment are the anatomical features: short, angulated fistulas that drain near the tricuspid valve and give rise to collateral branches close to the site where the occlusion is to be performed are contraindications for transcatheter closure. Moreover, surgery is generally preferred in multiple fistulas involving large branches, with a narrow, restrictive fistulous connection, that drain into a heart chamber or present an extreme tortuosity that impedes the distal delivery of the occlusion device. However, with the existing percutaneous closure devices, complete occlusion is achieved in>80% of cases, with few periprocedural complications,4,5 short hospital stays, rapid recovery, and excellent patient acceptance.
The progress in cardiac imaging studies, the growing experience of interventionists, the perfecting of percutaneous techniques, and the improvement in the materials employed make transcatheter closure of fistulas feasible in larger population groups.