Generally, percutaneous left atrial appendage (LAA) occlusion procedures are guided by 2-dimensional (2D) transesophageal echocardiography (TEE) and fluoroscopy. However, correct sizing of the required devices remains a challenging phase of the process. Furthermore, any advances able to minimize manipulation within the LAA and ease the implantation process would be welcomed.1
Patient-specific 3-dimensional (3D) heart models generated via 3D printing enable life-like replicas of image-based human anatomy, such as that obtained through multislice computed tomography, magnetic resonance imaging, and 3D echocardiography. This new technology impacts many medical specialties and has received increasing attention in the literature.2,3 Physical models of LAA occlusion are particularly relevant, because the anatomy is complex, and even if cutting-edge imaging is applied, gauging the interactions between the device and appendage is difficult.4
Our preliminary experience with patient-specific 3D-printed models of the LAA is presented herein, with the following objectives: a) to better appreciate LAA anatomy, location, and relationships; b) to aid in device sizing; and c) to optimize planning of LAA occlusion.
Ten patients with atrial fibrillation and with clinical indications for LAA closure were included in this initial analysis. In these 10 patients, multislice computed tomography images of the heart were segmented (ITK-SNAP freeware) and 3D meshes were created for 3D printing (220°C, 10mm/s, 0.1mm layer height, 0.8mm wall thickness) by an established method3 (Figure 1).
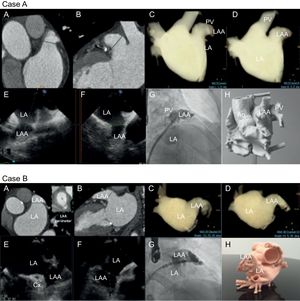
Multimodal imaging of LAA for cases A and B). A, B: cardiac measurements via computed tomography. C, D: computed tomography rendering of left atrial volume in working projections. E, F: transesophageal echocardiographic views (45° and 135°) showing landing zone. G: right anterior oblique cranial fluoroscopic projections; and H: 3-dimensional printed model. Ao, aorta; Cx, circumflex; LA, left atrium; LAA, left atrial appendage; PV, pulmonary vein.
Each LAA model required 500€ to produce and 24 to 48hours to complete. The printed models then served in the training/rehearsal phase. Expired LAA closure devices were deployed at this juncture to determine the following: a) ideal device, b) proper device size, and c) various orientations within the LAA conducive to either the success or failure of percutaneous closure. Device suitability was based on visually assessed anatomic deformation (in a specified LAA backdrop) and the feasibility of engagement within the flexible 3D atrial model (Figure 2).
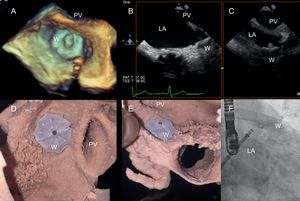
Ex vivo rehearsal phase and final outcome of left atrial appendage closure. A: 3-dimensional transesophageal echocardiogram. B, C: 2-dimensional transesophageal echocardiogram. D, E: 3-dimensional printed model; and F: fluoroscopic right anterior oblique cranial projection. W, Watchman device; LA, left atrium; PV, pulmonary vein.
As we observed, mean LAA diameters showed high degrees of correlation in imaging comparisons: computed tomography vs 2D-TEE (Pearson correlation coefficient [r], 0.98; P < .0001), fluoroscopy vs 2D-TTE (r, 0.92; P < .0001), and computed tomography vs fluoroscopy (r, 0.96; P < .0001). However, the mean values generated via 2D-TEE (21.9 ± 3.8mm) and fluoroscopy (22.2 ± 3.7mm) were significantly lower than those registered by computed tomography (23.1 ± 3.8mm) (P < .001 and P = .02, respectively). All 3D printing-derived device sizes nonetheless fully reflected those actually deployed, with no circumferential leakage. No adverse events were recorded at 30-day follow-up monitoring.
Of note, Budge et al.1 have reported a similar correlation between LAA measurements done by multislice computed tomography and by 2D-TEE. In addition, Otton et al.5 have shown that accurate approximations of device size (underestimated using TEE) are feasible with 3D-printed models. Finally, 2 instances of accurately sized and optimally implanted devices, based on a 3D-printed model (TEE and fluoroscopy estimates again low), have been documented by Pellegrino et al.6
From our perspective, the development of patient-specific 3D-printed physical models holds a wealth of promise in terms of proper device sizing under difficult circumstances, insights into LAA anatomic interrelationships, and viable device-specific technical strategies. There is also a potential to educate professionals accordingly, clarifying the aims and limitations of LAA occlusion. On the other hand, more clinical studies are needed to validate these uses, and logistics are of some concern. Although 3D heart models and 3D printing technology are now more widely available and more affordable, expenditures of time, money, and effort are still barriers to daily clinical use.
FUNDINGThis study received financial support from St Jude Medical, Inc (Minnesota, US). The sponsor had no role in designing the study, data collection, data analysis, data interpretation, or writing of the report. This research was cofinanced by Institute of Health Carlos III – FIS research grant number PI14/00180 from the Spanish Ministry of Science and Innovation.
CONFLICTS OF INTERESTI. Cruz-González is a clinical proctor for St Jude Medical and for Boston Scientific.
.
We are grateful to Drs Israel Valverde, Xavier Freixa and Oscar Alcade for their willing collaboration and review of this study.