Keywords
INTRODUCTION
Magnetic resonance imaging (MRI)—a noninvasive and precise imaging technique—has become much more widely used in recent years.1 It is increasingly common to request an MRI scan for patients who already have a cardiac pacing device, either a pacemaker (PM) or implantable carioverter-defibrillator (ICD). It is estimated that, after implantation of the device, each patient has a 50% to 75% probability of requiring an MRI scan during his or her life.2
The first data obtained in this field during the 1980s were disheartening. Serious complications, both electrical and clinical, were documented and there were even some reports of deaths.3,4 A contraindication for MRI was therefore established.
The current devices are smaller and made of materials with less risk of electromagnetic interference. In recent years, small safety studies have been performed.5-9 Although these have not been sufficient to change the recommended contraindications, they do suggest that no important complications occur if certain safety conditions are maintained.
We conducted a prospective study to assess the risks of MRI in patients with modern cardiac pacing devices.
For 14 months (October 2007 to December 2008), 29 patients with a cardiac pacing device for whom the MRI scan was indicated as the only available diagnostic test were included in the study. Patients with abandoned leads were not included. All 33 patients were informed of the possible consequences of the test and all gave their consent to the procedure.
Scans were performed in 5 patients with ICDs and 28 with PMs, 4 of whom were PM-dependent, defined as the absence of an independently stable ventricular rhythm above 40 beats/min.
Before and after the test, the devices were fully checked, recording the make, model, pacing mode, frequency of follow-up, battery stataus (impedance and/or voltage), pacing and detection thresholds and pacing impedance. Fifty-eight leads were analyzed (33 ventricular and 25 atrial). The pacing threshold was calculated for all cases, varying only the amplitude of the impulse, and so was expressed in volts.
In accodance with current guidelines, the pacing mode was changed from V00 to D00 in PM-dependent patients. Antitachycardiac therapies were deactivated in ICD patients. Bipolar detection was programmed, setting the output at double the pacing threshold and detection at half the amplitude of the wave detected, when the device so permitted.
All studies were performed with a 1.5T MRI apparatus (Siemens Magneton-Avanto), with and SAR limit <4 W/kg for the entire body surface.
During the test, electrocardiographic and pulse oximetry monitoring was carried out and verbal contact with the patient was maintained. As a safety measure, advanced cardiopulmonary resuscitation equipment was on hand in the MRI hall, along with an external cardioverter defibrillator and the programmer of the pacing device.
After the scan, a systematic questionnaire was administered with questions about pain, sensation of movement of the device, heat, or discomfort. In addition, the duration of the test was noted and the responsible radiologist was asked whether there were any defects in the image or any technical limitations.
The distribution of pacing mode, type of pacing device and type of MRI is shown in Table 1.
The quantitative parameters were analyzed using the t test for paired samples. In series with n<30 (battery impedance and voltage, atrial impedance, atrial and ventricular detection), a Mann-Whitney U test was used.
For the analysis of changes in the pacing threshold, the presence of variation was defined as a dichotomous variable. Subsequently, using the #c2 test, the possible relationship between the presence of variation and the paced chamber (atrium, ventricle) was analyzed, along with whether the site was infradiaphragmatic or supradiaphragmatic.
Qualitative data were expressed as percentages and quantitative data as mean (SD). Statistical significance was established at P<.05.
RESULTS
No clinical complications were reported. No patient reported pain, heat, discomfort, or sensation of movement of the device during the scan. In all cases, the scan was completed without any technical limitations; 1 case of an artifact was reported in the images obtained, during a cardiac MRI in a patient with a Vitality 2DR (Guidant) PM.
None of the electrical parameters changed significantly during the scan (Table 2).
In the 58 exposed leads, the pacing impedance was measured; the pacing threshold could not be determined in 3 atrial leads because the patient was in atrial fibrillation. In 11 cases (20%), there was a slight variation in the pacing threshold (a slight reduction in all but 3 cases, in which an increased threshold was observed); the largest change recorded was a decrease of 0.5 V in a ventricular device.
Of the 11 leads with a change in their pacing threshold, 5 were ventricular and 6 atrial (P=.271). Of the 11 studies in which a change in threshold was observed, 7 were infradiaphragmatic and 4 supradiaphragmatic (P=.023).
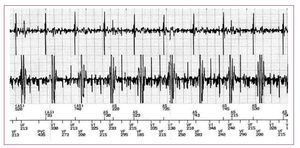
Detection failures were reported twice, once in an atrial lead and once in a ventricular one (Figures 1 and 2). The first case occurred during a brain MRI of a DDD-PM patient (EnPulse E2DR01 PM, Medtronic) and the second during cardiac MRI in an ICD patient (Guidant Vitality-2DR). In both cases, the radiofrequency pulses released during the scan were recorded as noise on the atrial and ventricular ECG, respectively. This noise signal was interpreted as atrial fibrillation in the first case—the mode change was activated automatically—and as ventricular fibrillation in the second one. According to the protocol, the antitachycardiac therapies had been deactivated beforehand, and so neither case was clinically relevant.
Figure 1. Atrial and ventricular electrocardiogram during the magnetic resonance imaging scan in a patient with an implantable cardioverterdefibrillator. A noise signal is observed in both channels, interpreted as ventricular fibrillation (VF) while the patient remained in stable sinus rhythm. The device did not apply any therapy as antitachycardia therapies had been deactivated beforehand.
Figure 2. Electrocardiogram of a DDD pacemaker patient during the magnetic resonance imaging scan. A noise signal can be seen in the atrial lead, which is interpreted as atrial fibrillation and the automatic mode switch is activated. During the scan, the patient was in sinus rhythm at all times according to the electrocardiogram.
Another event of note was the safety response of one of the PMs analyzed during a cholangiopancreatography scan; the device, a Clarity DDDR (Vitatron), began to pace in V00 mode at maximum frequency and output. After the MRI scan had been completed, the device could be reprogrammed to the prior pacing mode without any problems.
Finally, after 2 of the scans, a timeout to establish telemetric connection with the cardiac pacing device made it necessary to restart the programmer. After the connection had been reestablished, the lack of changes compared to the prior settings was confirmed, and after the MRI scan the testing could be performed without any complications.
DISCUSSION
We attempted to determine more exactly the risk that MRI scans represent for PM and ICD patients. We present a prospective study of 33 patients, conducted between October 2007 and December 2008. Thus the characteristics of the pacing devides and the MRI apparatus are similar to what is found in everyday clinical practice, although the findings should not be extrapolated to devices of different makes or models.
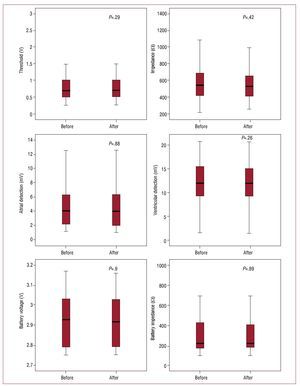
The devices studied responded safely to the electromagnetic interference caused by the MRI scan. No clinical event or significant change in the electrical parameters analyzed was observed (Figure 3). Likewise, there were no irreversible changes in device function. The events recorded were transient after reprogramming and did not represent any limitation in the operation of the devices or in performing the MRI.
Figure 3. Comparative graphic representation of the electrical parameters detected before and after magnetic resonance imaging. No statistically significant changes were observed.
The statistical result suggesting that a change in the pacing threshold is more likely after performing an infradiaphragmatic MRI scan should be interpreted with caution, given the low number of threshold changes detected (11 of 50 leads). Likewise, the low number of scans is a limitation for accurately describing small changes in the parameters analyzed, as well as for characterizing uncommon problems.
Our findings are in line with recently published series,10,11 and our conclusion also agrees with the general recommendations available at present.12 Given that larger prospective studies are lacking, the decision to conduct MRI scans in these patients should be considered on an individual basis. Current data suggest that, with cardiac monitoring and following some basic safety measures, MRI scans can be performed in PM and ICD patients who require such a test for clinical reasons, although it should be highlighted that the absolute safety of MRI scans in these patients cannot be guaranteed.
Correspondence: Dr. F. Buendía Fuentes.
Calderón de la Barca, 9, pta. 18, 46010 Valencia, España.
E-mail: franbuendia@gmail.com
Received April 30, 2009.
Accepted for publication July 21, 2009.