To the Editor,
Approximately 1% of patients in treatment with clopidogrel experience type I or IV hypersensitivity reactions consisting, in general, of rash or hematologic disorders (thrombocytopenia or neutropenia).1 More exceptionally, there have been reports of liver toxicity and systemic inflammatory response syndrome.2, 3 Recently, it has been proposed to replace this drug with prasugrel, a new third-generation thienopiridine,4 in situations in which antiplatelet therapy cannot be discontinued and it is therefore impossible to apply desensitization protocols.1, 5 To our knowledge, there are no reports in the literature of the reverse procedure in which a serious hypersensitivity reaction to prasugrel resolves after discontinuation of the drug and initiation of treatment with clopidogrel.
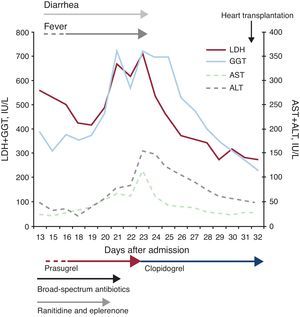
A 40-year-old women with a history of smoking was attended in a center other than our own for oppressive precordial chest pain. After a positive result in the ischemia detection test, a coronary angiogram was performed. This showed severe lesions in the left anterior descending artery and the diagonal coronary artery; revascularization was achieved by direct stenting, with a good initial angiographic outcome. At the time, antiplatelet therapy with acetylsalicylic acid was initiated (100mg/day) along with clopidogrel (300mg loading dose). A few hours after the procedure, the patient developed acute pulmonary edema with ST segment elevation in the anteroseptal leads. A repetition of the coronary angiogram showed complete acute thrombosis of the stent in the left anterior descending artery, and so the angioplasty procedure was repeated and clopidogrel was replaced by prasugrel (loading dose of 60mg followed by 10mg/day). Abciximab was added. Cardiogenic shock required orotracheal intubation and mechanical ventilation, as well as hemodynamic support with vasoactive drugs and intra-aortic balloon contrapulsation. The patient was then transferred to our hospital. On admission, the patient had a body temperature of 38.1°C, a blood pressure of 120/60mmHg, and a heart rate of 65 bpm. In the laboratory tests, of note were leukocytes 7.23×109/L (eosinophils 2.4%); aspartate transaminase 208 UI/L; alanine transaminase 93 UI/L; gamma glutamyl transpeptidase 240 UI/L; and alkaline phosphatase 170 UI/L. In addition to the vasoactive drugs and antiplatelet agents, treatment included ranitidine, sodium heparin, furosemide, and eplerenone. After initiating infusion of levosimendan, the hemodynamic status of the patient progressively stabilized, and she was extubated and the vasoactive drugs and intra-aortic balloon contrapulsation were withdrawn. At the same time, there was a transient improvement in her liver function tests. However, despite starting empirical antibiotic therapy with meropenem and linezolid, fever persisted in the days that followed, with small daily oscillations in temperature (<1°C) and transient remission after administration of antipyretics. Microbiological study were sterile or negative; these included serial blood cultures, urine cultures, and cultures of endotracheal aspirate; serology for hepatotropic viruses and the human immunodeficiency virus cytomegalovirus antigenemia; and the Mantoux skin-prick test. Similarly, full autoimmune testing was negative. The fever did not remit after changing venous and arterial catheters or suspending various drugs (ranitidine, heparin, and eplerenone). After 12 days, the number of stools began to increase (6-8 per day), with no pathological contents and also with negative etiologic study (coproculture and detection of Clostridium difficile toxin). Both the leukocyte count and acute-phase reactants (C-reactive protein and procalcitonin) remained within normal limits at all times, although there was a progressive change in the liver function tests (day 23 after admission: aspartate transaminase 115 UI/L, alanine transaminase 155 UI/L, and gamma glutamyl transpeptidase 723 UI/L), and relative eosinophilia appeared (day 19, eosinophils 9.7%). Abdominal ultrasound did not reveal any liver or bile duct abnormalities. Finally, faced with suspected drug-mediated hypersensitivity reaction, it was decided to suspend prasugrel and start clopidogrel without making any other changes to therapy. In the first 24h after the change, the fever and diarrhea remitted, and there was a progressive return to normal liver function tests and leukocyte differentials (Figure). After 7 days, the patient had a second episode of low cardiac output that required administration of vasoactive drugs. This time, there were no accompanying abnormal laboratory results or fever. As she had a left ventricular ejection fraction of 5% to 10%, with no possibility of revascularization, she was included on the waiting list for heart transplantation. This procedure was performed on day 32 after admission, without the reappearance of symptoms in the subsequent follow-up. We reported the case to the Pharmacovigilance Center of the Community of Madrid.
Figure. Clinical course and laboratory changes during admission. ALT, alanine transaminase; AST, aspartate transaminase; GGT, gammaglutamyl transpeptidase; LDH, lactate dehydrogenase.
Although severe hypersensitivity reactions to clopidogrel have been reported in the literature,2, 3, 5 we were unable to find any cases in which prasugrel was implicated (searching MEDLINE with the MeSH [Medical Subjects Headings] terms “prasugrel” and “fever” or “hepatitis” or “allergy” or “eosinophilia”). The fever in our patient was persistent with a “plateau,” associated with eosinophilia, diarrhea, and worsening liver function despite hemodynamic normalization and, in the absence of leukocytosis or elevated acute-phase reactants, was grounds for suspicion of a hypersensitivity reaction.3 However, we did not observe any changes in the body temperature profile or laboratory tests after withdrawing drugs that have occasionally been linked to hypersensitivity reactions, such as ranitidine or heparin. In addition, the administration of furosemide continued throughout admission, including after symptoms had remitted. Any involvement of this drug can therefore reasonably be ruled out. Thus, as alternative causes were ruled out and given the rapid resolution of the clinical manifestations and laboratory parameters after discontinuation of prasugrel, a relationship with this drug can be established as “probable” according to the 16-point Maria and Victorino scale.6 Nevertheless, for ethical and safety reasons, we did not rechallenge, which would have provided a more definitive diagnostic criterion for hypersensitivity.3, 6 Despite their structural similarities, cross-hypersensitivity between clopidogrel and prasugrel have yet to be reported, although patients with allergy to thienopyridines were excluded from clinical trials performed with prasugrel.1 In short, this case report illustrates the need to include hypersensitivity reaction to prasugrel in the differential diagnosis in certain clinical circumstances (persistent fever, eosinophilia, diarrhea, and hepatitis). It also indicates, for the first time, that replacement with clopidogrel could be a safe option in patients who must continue antiplatelet therapy. In any case, the evidence of an isolated report should be treated with caution. In view of the impossibility of establishing a solid recommendation, it would be desirable to report future cases of the management of hypersensitivity reactions to thienopyridines.
FundingMario Fernández Ruiz received a grant from the Fundación para la Investigación Biomédica of the Hospital Universitario 12 de Octubre. Francisco López Medrano received a grant from the Fundación Mutua Madrileña.
.
Corresponding author: mario_fdezruiz@yahoo.es