Transcatheter aortic valve implantation (TAVI) has become consolidated in recent years as a safe and effective alternative treatment for patients with severe symptomatic aortic stenosis with high surgical risk.1 The most commonly used scales for calculating preoperative surgical risk are the EuroSCORE (European System for Cardiac Operative Risk Evaluation)—the preferred scale in our setting—and the STS (Society of Thoracic Surgeons) scale.1 However, these risk scales do not include some conditions, such as porcelain aorta,2 frailty, and liver cirrhosis, which also have increased surgical risk.1
Patients with cirrhosis who undergo cardiac surgery are at increased risk of perioperative mortality and morbidity.3 These patients are more likely to have serious coagulopathy and multiorgan dysfunction associated with cardiopulmonary bypass, and treatment with TAVI could be a therapeutic alternative.4 There are no conclusive data regarding the role of TAVI in patients with severe aortic stenosis and liver cirrhosis.5 The aim of this article is to describe the results in a series of such patients treated with TAVI.
Between June 2008 and September 2011, 10 consecutive patients with severe aortic stenosis and liver cirrhosis were treated with TAVI. The patients came from 3 Spanish hospitals: Hospital Virgen de la Victoria in Malaga (n=7), Hospital Universitario Central de Asturias in Oviedo (n=2), and Complexo Hospitalario Universitario in Santiago de Compostela (n=1). Prior to implantation, the patients were evaluated by a multidisciplinary team1: 7 patients were ineligible for surgery, and 3 refused to undergo surgery. We assessed surgical risk according to the logistic EuroSCORE, and the degree of severity of cirrhosis according to the Child-Pugh International Classification.6 This classification has 3 grades, A, B, and C, from lowest to highest severity of liver failure.6 The self-expanding CoreValve® prosthesis (Medtronic, Minneapolis, Minnesota, United States) was implanted in all patients. Follow-up was conducted until June 30, 2014, via direct contact with the patients themselves or close relatives. The primary objective of the study was to analyze mortality due to any cause. We performed a basic descriptive statistical analysis and survival analysis (Kaplan-Meier) using SPSS (Chicago, Illinois, United States).
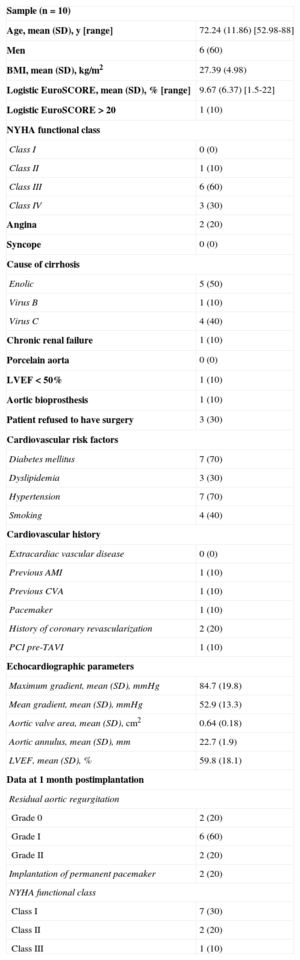
The baseline characteristics of the population are shown in the Table. Notably, the mean age was lower than in other series. The mean (SD) EuroSCORE was 9.7 (6.4) and 1 patient showed a EuroSCORE > 20. Ninety percent of patients were in functional class III or IV. One patient had chronic renal failure, another had left ventricular ejection fraction < 50%, and another was treated for deterioration of an aortic valve bioprosthesis. Liver cirrhosis classification was Child-Pugh grade A in 7 patients (including the 3 patients who refused surgery), A5 in 3 patients, A6 in 2 patients, grade B in 2 patients (B7 and B9), and grade C in 1 patient.
Baseline Characteristics of the Sample
| Sample (n=10) | |
| Age, mean (SD), y [range] | 72.24 (11.86) [52.98-88] |
| Men | 6 (60) |
| BMI, mean (SD), kg/m2 | 27.39 (4.98) |
| Logistic EuroSCORE, mean (SD), % [range] | 9.67 (6.37) [1.5-22] |
| Logistic EuroSCORE > 20 | 1 (10) |
| NYHA functional class | |
| Class I | 0 (0) |
| Class II | 1 (10) |
| Class III | 6 (60) |
| Class IV | 3 (30) |
| Angina | 2 (20) |
| Syncope | 0 (0) |
| Cause of cirrhosis | |
| Enolic | 5 (50) |
| Virus B | 1 (10) |
| Virus C | 4 (40) |
| Chronic renal failure | 1 (10) |
| Porcelain aorta | 0 (0) |
| LVEF < 50% | 1 (10) |
| Aortic bioprosthesis | 1 (10) |
| Patient refused to have surgery | 3 (30) |
| Cardiovascular risk factors | |
| Diabetes mellitus | 7 (70) |
| Dyslipidemia | 3 (30) |
| Hypertension | 7 (70) |
| Smoking | 4 (40) |
| Cardiovascular history | |
| Extracardiac vascular disease | 0 (0) |
| Previous AMI | 1 (10) |
| Previous CVA | 1 (10) |
| Pacemaker | 1 (10) |
| History of coronary revascularization | 2 (20) |
| PCI pre-TAVI | 1 (10) |
| Echocardiographic parameters | |
| Maximum gradient, mean (SD), mmHg | 84.7 (19.8) |
| Mean gradient, mean (SD), mmHg | 52.9 (13.3) |
| Aortic valve area, mean (SD), cm2 | 0.64 (0.18) |
| Aortic annulus, mean (SD), mm | 22.7 (1.9) |
| LVEF, mean (SD), % | 59.8 (18.1) |
| Data at 1 month postimplantation | |
| Residual aortic regurgitation | |
| Grade 0 | 2 (20) |
| Grade I | 6 (60) |
| Grade II | 2 (20) |
| Implantation of permanent pacemaker | 2 (20) |
| NYHA functional class | |
| Class I | 7 (30) |
| Class II | 2 (20) |
| Class III | 1 (10) |
CVA, cerebrovascular accident; EuroSCORE, European System for Cardiac Operative Risk Evaluation; LVEF, left ventricular ejection fraction; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; BMI, body mass index; NYHA, New York Heart Association; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
Unless otherwise indicated, data are expressed as No. (%).
The procedure was performed under heavy sedation, under general anesthesia in 9 patients, and using mechanical ventilation in 1 patient. The percutaneous femoral access route was used in all patients, with subsequent percutaneous closure.
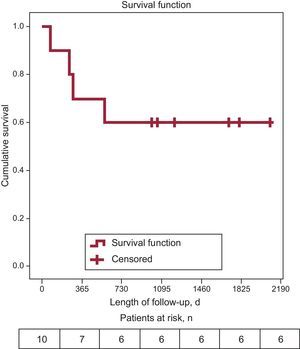
No deaths occurred during hospitalization or during the 30 days following implantation. One patient had major bleeding complications secondary to perforation of the femoral artery, which was treated by implantation of a coated stent. One patient required implantation of a second valve. The mean (SD) hospital stay was 10.4 (6.5) [6-22] days. Four patients died during follow-up (median 1031 days; interquartile range, 268-1737). One patient (Child-Pugh B9) died at 250 days after implantation due to refractory heart failure (EuroSCORE 22%). The other 3 deaths were of noncardiac causes: 1 (Child-Pugh A5) at 275 days due to head injury with intracranial hemorrhage; the other 2 at 71 (Child-Pugh C) and 581 (Child-Pugh A5) days due to multiorgan failure secondary to severe sepsis as a result of spontaneous bacterial peritonitis. Overall survival (Figure) was 60%, with an estimated median survival of 1373.5 days (95% confidence interval, 822.6-1924.4).
One patient, who had Child-Pugh B7 at the time of implantation, was ultimately added to the waiting list for a liver transplant 2 years after the TAVI, due to progression of liver disease.
In this series of patients with severe symptomatic aortic stenosis associated with liver cirrhosis, percutaneous TAVI treatment was effective. The success rate of the procedure was high and did not differ from that reported in larger series without associated liver cirrhosis. In this regard, we note the low rate of complications and mortality associated with the implant and during the hospital stay, although this sample did not have high surgical risk, other than that due to cirrhosis.
From a cardiovascular point of view, the long-term outcome of these patients was also favorable, since there was only 1 death due to this cause.
In conclusion, treatment with TAVI can be considered as an effective therapeutic alternative, even as a bridge to liver transplantation in patients with severe aortic stenosis and liver cirrhosis.