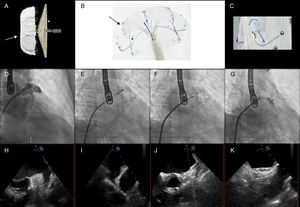
Percutaneous closure of the left atrial appendage (LAA) is a therapeutic alternative to oral anticoagulation for ischemic stroke prevention in patients with nonvalvular atrial fibrillation.1,2
Currently, the 2 most commonly used devices for percutaneous LAA closure are the Watchman (Boston Scientific) and the Amulet (St Jude Medical). Although the success rates of the procedure are increasingly higher and the complication rate is decreasing,3–5 there are still some technically complicated cases in which devices with a new design could facilitate implantation.
The LAmbre device (Lifetech Scientific [Shenzhen] Co Ltd) recently obtained the CE mark. To our knowledge, no case series has yet been described that shows the feasibility and safety of LAA closure with this device.
The main distinguishing features of the LAmbre device6 (Figure) are the 8- to 10-Fr delivery sheath with a double distal curve of 45 × 30° (compared with 12-14 Fr and a distal sheath angle of 45 × 45° for the Amulet and the Watchman), the option of different cover sizes for the same umbrella size (in the Amulet device, each lobe size comes with a fixed disc size), the distance between the cover and umbrella of 4mm but with a convex appendicular surface of the cover, and the double stabilization system (hooks and U-shaped anchors that attach in the trabeculae and pectinate muscles).
LAmbre device and fluoroscopic and echocardiographic images of device implantation. A: LAmbre device (the arrow indicates the umbrella, and the asterisk, the cover). B: detail of the double stabilization mechanism (the arrow indicates the hooks, and the asterisk, the U-shaped anchors). C: detail of the anchors. D: initial angiography. E: umbrella deployment. F: cover deployment. G: final result. H and I: baseline transesophageal echocardiography. J and K: final transesophageal echocardiography.
The study included all consecutive patients who underwent percutaneous LAA closure with the LAmbre device in 3 centers between October and December 2016. The procedure was performed under fluoroscopy and transesophageal echocardiographic guidance as per the standard technique for other devices; the landing zone was measured at 15mm from the LAA ostium.
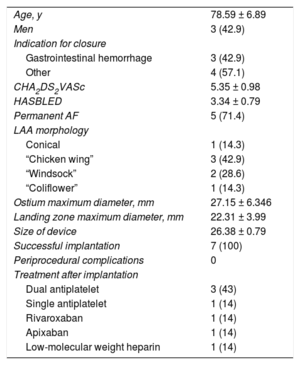
Seven patients were included in the study. The population and procedure characteristics are shown in Table. Successful LAA closure with the LAmbre device was achieved in all patients, despite complex anatomy (28% of the LAAs had > 10mm difference in diameter between the landing zone and the ostium, and 42% had “chicken wing” morphology). There were no cases of device embolization, pericardial effusion, stroke, acute myocardial infarction, or death during the procedure. There were no complications on echocardiography prior to discharge. Treatment after implantation followed the standard practice of each center for other devices (Table 1).
Baseline Population and Procedure Characteristics
| Age, y | 78.59 ± 6.89 |
| Men | 3 (42.9) |
| Indication for closure | |
| Gastrointestinal hemorrhage | 3 (42.9) |
| Other | 4 (57.1) |
| CHA2DS2VASc | 5.35 ± 0.98 |
| HASBLED | 3.34 ± 0.79 |
| Permanent AF | 5 (71.4) |
| LAA morphology | |
| Conical | 1 (14.3) |
| “Chicken wing” | 3 (42.9) |
| “Windsock” | 2 (28.6) |
| “Coliflower” | 1 (14.3) |
| Ostium maximum diameter, mm | 27.15 ± 6.346 |
| Landing zone maximum diameter, mm | 22.31 ± 3.99 |
| Size of device | 26.38 ± 0.79 |
| Successful implantation | 7 (100) |
| Periprocedural complications | 0 |
| Treatment after implantation | |
| Dual antiplatelet | 3 (43) |
| Single antiplatelet | 1 (14) |
| Rivaroxaban | 1 (14) |
| Apixaban | 1 (14) |
| Low-molecular weight heparin | 1 (14) |
AF, atrial fibrillation.
Values are expressed as No. (%) or mean ± standard deviation.
These excellent results could be explained by our growing experience in percutaneous LAA closure. However, we must consider the LAmbre design: the smaller sheath size could reduce the number of vascular complications, the 45 × 30° double curve could reduce the risk of sheath folding if the operator attempts to reach the more anterior lobes even with medial transseptal puncture (although the ideal transseptal puncture zone for this device is inferior and posterior), the double stabilization system may potentially reduce embolization and facilitate implantation by reducing the number of recaptures, and–in our opinion the most important distinguishing feature–the availability of “special” devices with large covers for small umbrellas (eg, umbrella/cover of 22/34 or 26/38mm) could facilitate implantation in “chicken wing” anatomies with a very short implantation zone or conical LAAs with a large size difference between the ostium and the medial part of the LAA.
The greatest limitation of this study is its observational nature and the small number of patients included. When interpreting the excellent results, it must be taken into account that the procedures were performed in experienced centers. Because of the low number of patients and the absence of mid- and long-term follow-up, we cannot attribute the lack of complications directly to the design of this new device. The excellent results from large-scale studies of the most widely used devices at present (Amulet and Watchman) mean we must reserve judgment on the potential benefits of this new device until there are larger complete registries.
Nonetheless, to our knowledge, this is the first published series on LAA closure with the LAmbre device to show its feasibility and safety. The design and distinguishing features of this device could be of help in patients with complex anatomy.
.