Keywords
PROGRESS IN INTERVENTIONAL CARDIOLOGY: FROM BALLOON ANGIOPLASTY TO DRUG-COATED STENTS
Since the first percutaneous transluminal angioplasty was performed in a human patient in 1977,1 interventional cardiology has been the therapeutic modality that has advanced more rapidly than any other form of treatment in the field of cardiovascular disease. This technique initially produced results that would not meet current standards. In the first studies, the reported success rate was 86%-88%, with a restenosis rate of 30%-40%.2-8 For more than a decade, this technique achieved widespread popularity in clinical practice, despite the fact that the rates of acute or subacute occlusion reached as much as 8%.9 Angioplasty continued to be used, sometimes with disappointing results, particularly in complex lesions, and for years randomized studies in which angioplasty was compared with other treatment modalities were unavailable (the first randomized trial was performed 10 years after its introduction in the context of acute myocardial infarction10). To address the complications associated with the technique and reduce the restenosis rate, new devices were developed at the end of the 1980s that, from a theoretical perspective, offered certain advantages over balloon dilatation. Devices were designed as alternatives to the balloon that, instead of flattening and fracturing the plaque, resected or pulverized it, removing the obstruction from the stenotic segment. This was the basis of atherectomy, which in its time increased the therapeutic arsenal of the catheterization laboratory. The most widely used atherectomy techniques were directional atherectomy (Simpson AtheroCath), rotational atherectomy (Rotablator), and transluminal extraction coronary (TEC) atherectomy.
Directional Atherectomy
The technique of directional atherectomy was introduced by Simpson in 1985 as an alternative to balloon angioplasty in the treatment of atherosclerosis of the peripheral arteries. Subsequently, the same technique was applied to coronary disease with notable initial success. At that time, numerous publications demonstrated the usefulness of this therapeutic modality, which allowed resection and removal of the atheroma plaque.11-13 This initial enthusiasm for atherectomy was diminished by the difficulty of introducing a rigid device through a thick 10-11 Fr guide catheter that had little flexibility and was difficult to navigate. In addition, randomized studies comparing the results of this technique with those of balloon angioplasty did not reveal any beneficial effects. The first of the large randomized studies was the CAVEAT study,14 in which more than 1000 patients were included in order to compare directional atherectomy with balloon dilatation. This study found that atherectomy generated a greater increase in vessel caliber than angioplasty. However, the procedure was associated with a higher rate of complications, without significant differences in terms of clinical recurrence or restenosis. The lack of significant differences in favor of directional atherectomy in this study led some researchers to consider the possibility that these results could be due to problems in the technical realization of the atherectomy. Consequently, 2 new large randomized studies were designed in which balloon angioplasty was compared with an optimized atherectomy procedure. These studies (OARS and BOAT) demonstrated a good immediate result in terms of increased lumen diameter but without long-term clinical advantages when compared with patients treated with balloon angioplasty.15,16 Consequently, the use of this technique has now dropped considerably in Spain.17
Rotational Atherectomy (Rotablator)
In contrast to directional atherectomy, which cuts and collects the atherosclerotic material, the Rotablator produces an abrasion of the atheroma plaque, pulverizing it into microparticles (generally <5µ in diameter) that embolize to the coronary capillaries and are phagocytized by macrophages, without causing significant embolic occlusion of the small vessels. The device consists of an elliptical metal burr with a surface covered in diamond chips of 5-10 µ diameter mounted at the distal end of a flexible drive shaft that is connected to a console from which the rotation speed is controlled and monitored. The rotation speed ranges from 140 000 to 190 000 revolutions per minute. Like directional atherectomy, this modality was taken up enthusiastically for use in lesions considered unsuitable for angioplasty. In the first few years following its introduction, this device was used in ostial, calcified, long, and diffuse lesions, and lesions in which dilatation is not possible, obtaining a primary success rate of 91%-95% with a mortality rate of 0%-3.3%.18,19 Currently, although not as many randomized studies have been performed as in the case of directional atherectomy, there continues to be a niche in our therapeutic arsenal for rotational atherectomy.17 This niche relates to highly calcified lesions that are not amenable to dilatation, and those in which it is impossible to pass a device once the lesion has been crossed by the coronary guidewire. Although these lesions are infrequent, they cannot be resolved in any other way using percutaneous techniques.
Other atherectomy modalities have fallen into disuse due to the development of more effective devices.
Coronary Stents
A significant advance came with the introduction into clinical practice of the Palmaz-Schatz stent20 in 1991. In addition to sealing the plaque and any dissections that may have been produced during balloon dilatation, this device also created a large lumen in the treated vessel. However, the fact that it was a metallic device meant that it was not without complications. The possible appearance of thrombi inside the prosthesis and stent thrombosis were the feared complications during the first 3 years following its introduction.20-22 To address these complications, it was initially recommended that aggressive antithrombotic therapy with unfractionated heparin should be used during the procedure, followed by continuous perfusion until oral anticoagulants became effective.21,22 Despite this aggressive anticoagulation, stent thrombosis occurred in 5% to 7% of cases, and an appreciable incidence of hemorrhagic complications was seen.20-23 It was not until 1994, with the arrival of new antithrombotic regimens consisting of antiplatelet treatment using a combination of ticlopidine and aspirin (with or without low molecular weight heparin), that these complications were reduced to less than 1%-2%.23-27 From this point onwards, the stent was considered the most effective device for use in percutaneous coronary revascularization. Subsequently, the industry began to develop a series of improved techniques to facilitate implantation of the device. The stent, which initially came separately, was later manufactured mounted on the balloon, thereby enhancing the safety of the procedure. Subsequently, the reduced profile of the balloon-stent system, along with the production of different lengths and diameters, allowed it to be used in a series of lesions that had previously been considered unsuitable for percutaneous treatment. By around the middle of the 1990s, the rate of subacute occlusion had been reduced to less than 1%-2%23-27 and that of restenosis from 30%-40% down to 20%-30%.28-30 From then on, the scientific community focused its efforts on reducing the rate of restenosis. Many drugs with a theoretical antimitotic and antiproliferative capacity were tested, and although reductions in the degree of neointimal proliferation were obtained in experimental animals, it did not translate into similar results in humans.31 Thus, 10 years elapsed between the introduction of the stent and the identification of a means with which to reduce the rate of restenosis. In the meantime, the techniques associated with stent implantation were improved with the introduction and development of thrombectomy and distal protection devices. In lesions containing a large thrombus, the risk of detachment during stent implantation could compromise the success of the procedure.32,33 To reduce the incidence of this complication, thrombectomy devices were developed that have continued to be perfected; currently, some highly simplified devices are available that essentially consist of a hollow tube connected to a vacuum, alongside other very effective ones that require more complex technology.34
Without doubt, the most important advance in the decade following the introduction of the stent and antithrombotic regimens has been the introduction of drug-coated stents; a chapter will be dedicated to the subject of drug-coated stents in this Update on myocardial revascularization. Table 1 provides a summary of the main events that occurred in the last decade associated with the improvement of percutaneous treatment, with the dates of their introduction that would correspond to an average hospital in Spain.
CURRENT RESULTS AND COMPLICATIONS
To date, the results of interventional coronary procedures depend to a large degree on the type of lesion. As early as 1988, the American Heart Association published guidelines for coronary angioplasty in which types of lesion were classified in terms of 3 risk categories35 (Table 2). Type A lesions had a probability of success of more than 85% and a low risk of acute occlusion. Type B lesions had a probability of success of between 60% and 85% and a moderate risk of abrupt occlusion. Finally, type C lesions had a probability of success of less than 60% and a high risk of abrupt occlusion following the procedure. More recently, in 2001, in the era of the stent, the same risk classification was maintained.36 However, the probability of success in each of the risk groups has increased considerably with the use of currently available devices.
Low-Risk Lesions (Type A)
The probability of success in low-risk lesions was enhanced by the arrival of the stent28,29 and improved in the era of modern antithrombotic regimens.23-27 The safety of the procedure in this type of lesion has continued to improve in recent years with the introduction of better techniques, and the probability of success is now greater than 95%. With the advent of rapamycin-coated stents, the risk of restenosis has been reported to be close to 0%.37
Moderate-Risk Lesions (Old Type B)
In the moderate risk group of lesions, it is necessary to individualize the results of intervention according to the subtype. In moderately long lesions (10-20 mm), eccentric lesions, lesions that display moderate calcification, an irregular contour, or moderate tortuosity of the proximal segment, or moderately angulated segments, the primary success rates increased with the arrival of the conventional stent, as in the case of type-A lesions.28,29 The main problem with balloon angioplasty was occlusive dissection, which could be resolved in these types of lesion by sealing through implantation of additional stents. Consequently, in these groups the primary success rate of 60%-85% for balloon angioplasty improved from around the middle of the 1990s onwards.
Thrombotic Lesions
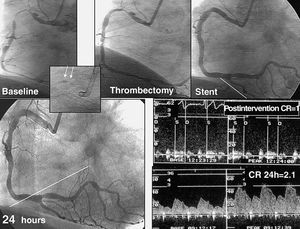
With first-generation balloon angioplasty, thrombotic lesions exhibited a high incidence of acute occlusions occurring during the procedure or in the first 24-48 hours. Although some authors proposed the use of adjunct thrombolytic drugs, it did not prove to be effective in reducing periprocedural complications.38 In contrast to other type B lesions, interventional cardiologists were initially reticent about using stents in these situations since it involved the use of a metallic and, therefore, thrombogenic structure. It was suggested that the use of a stent would not resolve the thrombotic process that accompanies this type of lesion. However, in practice, the reverse proved to be true: excellent results were obtained with the use of stents in the presence of thrombi.32,33 The arrival of new antithrombotic regimens and glycoprotein IIb/IIIa inhibitors made the procedure much safer.39 Again, these advances, along with the use of thrombectomy devices (Figure 1),40 have led to much higher success rates; consequently, we have moved from a probability of success of 60%-85% to currently more than 95%, with a risk of complications of less than 5% in this type of lesion.
Figure 1. Example of a lesion with a large thrombus in the context of unstable angina. Excellent results are observed by angiography following thrombectomy and implantation of stents. Recovery of the coronary reserve (CR) takes 24 hours. Arrows indicate the position of the thrombus in the aspiration catheter, shown using a contrast dye.
Ostial and Bifurcation Lesions
In the era of balloon angioplasty, results in ostial and bifurcation lesions were disappointing, with a high incidence of complications. Vetrovec et al41 described an incidence of sidebranch occlusion of up to 27%, in which the occlusion was caused by dissection or plaque displacement. At that time, many surgeons avoided tackling these types of lesion, despite the fact that some techniques had been described, such as the kissing balloon42 or protection with double guidewires.43 The arrival of the conventional stent caused a revolution in the treatment of these types of lesion and multiple strategies were described for stent implantation, many of which are still in use. The results that were obtained during that period (1994-2001), although acceptable, were a long way from what we can expect to obtain nowadays. Thus, the probability of major events at 6-month follow-up (infarct, repeat revascularization, or mortality) ranged from 50% in patients who received stents in the main and side branches to 25%-38% in those who received a stent in the main branch and angioplasty in the side branch.44-47 In the last 2 years, results have improved notably with the arrival of drug-coated stents. In recent studies,48-50 major events with rapamycin coated stents at 6-month follow-up have been reduced to levels as low as 7%-19%, according to the strategy employed. These results represent a 2- to 3-fold reduction in major events compared with previous techniques, and herald a new era in the treatment of major bifurcations.
Low-Risk Lesions (Type C)
Again, we will analyze the results of percutaneous interventional cardiology according to the type of lesion in the low-risk group.
Diffuse Lesions (>20 mm in Length)
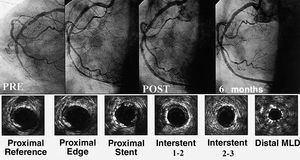
Diffuse lesions were also considered unsuitable for treatment with balloon angioplasty. Initial experience with stents was discouraging due to the high incidence of subacute stent thrombosis and the high percentage of restenosis.51 Improved implantation techniques, new antithrombotic regimens, and new designs of long stents improved the safety of treatment in these long lesions. Various implantation strategies competed to obtain the best results: implantation of a single long stent, implantation of various stents with or without overlap, and the technique of spot stenting. However, no clear benefit was shown by any one of the techniques.52-59 Use of the conventional stent in diffuse lesions (>20 mm) resulted in a probability of event-free survival at 1 year of 80%. However, in high-risk groups (vessel diameter of <3 mm), the results were worse, with a probability of event-free survival at 1-year follow-up of 55%.59 The probability of restenosis also differed widely according to different authors and it was reported at up to 73% restenosis in patients with a postimplantation stent area of <5 mm2 determined by ultrasound.60 The arrival of drug-coated stents led to a significant improvement in medium-term results (Figure 2). Recent studies have reported an incidence of major events at 6-10 months of 8%-12%, with a restenosis rate of 12%-14% in these types of lesion.61,62 Again, these results are very favorable when compared with earlier reports.
Figure 2. Occluded right coronary artery with diffuse disease reconstructed with multiple rapamycin-coated stents. At 6-month follow-up, the artery remains permeable, with minimal neointimal proliferation seen by echocardiography (bottom panels). MLD indicates minimum lumen diameter
Chronic Occlusion for More Than 3 Months
The approach to treating chronic occlusion present for more than 3 months consists of 2 clearly differentiated parts: the first involves crossing the occluded segment with a guidewire, while the second involves dilatation of the occlusion and use of a device to achieve a large lumen and maintain it open in the long term. The first part of the procedure is perhaps the area in which least progress has been made in the last 20 years. Nevertheless, once the lesion is crossed, technical advances have indeed improved results, to a similar extent as seen with the lesions described above. The profile of the new balloons, the characteristics of the mounted stents, and most recently, the arrival of drug-coated stents represent the greatest improvements in the treatment of this subtype of lesions. Suero et al63 demonstrated that the prognosis of patients with chronic occlusion and treatment success is better than in patients who do not undergo recanalization. Consequently, efforts have been made to improve the success rate of crossing chronic occlusions and special guidewires have been developed with hard tips that are able to penetrate the obstructed segment. The initial success in crossing chronic occlusions with conventional guidewires was between 42% and 70%,64 depending on the type of occlusion, the length of time that it had been present, and the length of the obstructed segment. Although the industry has designed specific guidewires with which to cross occlusions, no ideal model has been obtained that serves in all cases. Perhaps the greatest advance in this field has been the development of the Safe-Cross radio frequency guidewire. This guidewire applies radio frequency at its distal tip to achieve better penetration, and it is supplied with a reflectometry system that uses light energy to inform the operator about the position of the tip in order to avoid any type of perforation or penetration of the arterial wall due to misdirection of the guide. The success rate of this new device in cases that have failed using conventional guidewires is approximately 54%.65 Once the occlusion is crossed, progress has been more rapid since the time of balloon angioplasty. The rate of restenosis dropped from 60%-70% down to 20%-30% with the use of the conventional stent.66-69 Drug-coated stents have led to a further reduction in the rate of recurrence once the occlusion is crossed. The probability of restenosis using these stents has been reduced to 9%-13%,70,71 with a requirement for repeat revascularization of the treated lesion in less than 6% of cases.
Severely Angulated Segments
The arrival of mounted stents, which are much more flexible, and of high-support guidewires have allowed severely angulated lesions to be treated with greater primary success and lower risk, since if distal dissections are produced they can be sealed with new stents.
Excessive Tortuosity of the Proximal Segment and Impossibility of Protecting Adjacent Major Branches
The 2 most extreme situations that currently persist without a definitive solution from a percutaneous perspective are excessive tortuosity of the proximal segment and the impossibility of protecting adjacent major branches. If the operator is unable to maneuver the stent into the lesioned segment or once it is dilated with the balloon there is a risk of not resolving severely compromised blood flow in the region. The coronary arteries sometimes present tortuosities of more than 360° or side branches with curves that are practically impossible to cross using current systems. In these cases, although infrequent, there remains a low primary success rate and the possibility even remains that it is impossible to access the lesion in order to perform the dilatation.
Degenerated Bypass Grafts With Unstable Lesions
Degenerated bypass grafts present a dual difficulty for percutaneous treatment: the first is the possibility of embolism caused by the unstable material of the plaque leading to myocardial infarct, and the second is the high rate of late recurrence, either due to restenosis or progression into another area. Recently, Keeley et al72 reported their experience in the treatment of 1142 lesions in degenerated grafts. The authors observed an incidence of major events in hospital of 13%, including 8 deaths, with an incidence of late events of 54% (9 deaths, 9 non-Q wave infarcts, and 36% repeat revascularization of the treated lesion). Despite numerous technological and pharmacological advances, percutaneous intervention in degenerated grafts is associated with a high incidence of periprocedural complications and major events during follow-up. The use of thrombectomy34,40 or distal embolic protection devices73 can protect the distal bed from fragments of thrombus that may detach and reduce the risk of embolic infarct. At the same time, drug-coated stents can reduce the probability of restenosis; however, disease progression in untreated segments makes it difficult to resolve this type of lesion percutaneously.
BROADENING OF THE INDICATIONS
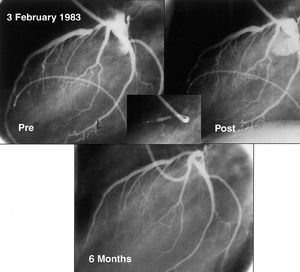
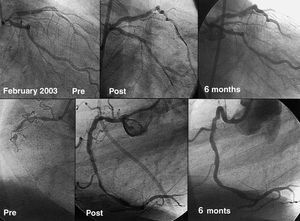
The initial indications for percutaneous interventional cardiology were limited to circumscribed lesions in patients with single vessel disease (Figure 3) and with good ventricular function. This ensured a high success rate and, at the same time, any complications that arose were better tolerated than in patients with depressed ventricular function. With the advances and improvements mentioned for the treatment of all types of coronary lesions, this initial indication was broadened in line with the increasing experience of cardiologists and the development of new devices. In this way, interventional cardiology has gradually tackled more complex lesions, higher risk patients, and more extensive coronary disease (Figure 4). Although the current therapeutic arsenal is powerful, there are still some types of lesion and patients that cannot be treated percutaneously. These relate to situations in which it is impossible to gain access to the target lesion, either as a result of chronic occlusion or severe tortuosity and calcification that impede maneuvering of the stent to the desired position. With the exception of these situations, interventional cardiology is highly successful in the treatment of the majority of patients with coronary disease. The broadening of the indications has been subjected to comparative studies with surgical revascularization. From the outset, a number of randomized studies have compared percutaneous revascularization with surgery.74 However, the advances in interventional cardiology have been so rapid that when the long-term results of these studies became available for publication, the interventional techniques used had already become obsolete. Consequently, 2 revascularization modalities continue to be used in parallel without evidence of any clear benefit of one or the other in terms of survival. However, since percutaneous methods are simpler, less invasive, and can be employed immediately following diagnosis, many interventional laboratories have decided to routinely treat multivessel disease and lesions of the left main coronary artery.
Figure 3. Angiograph of the first angioplasty performed in the Reina Sofía Hospital, Cordoba, on 3 February 1983. It shows an easily accessible proximal lesion in a straight segment that is concentric, has smooth edges, and fulfills all the criteria of a favorable lesion for interventional treatment. An acceptable result is seen by angiography that is maintained on follow-up at 6 months.
Figure 4. Example of a patient recently revascularized in our hospital 20 years after the first angioplasty was performed. The patient presents a severe bifurcation lesion in the left main coronary artery that is severely calcified. The right coronary artery also contains an occlusion that is more than 3 months old. The bifurcation of the artery is resolved by implantation of a rapamycin-coated stent close to the anterior descending artery and balloon angioplasty at the origin of the circumflex artery. The occlusion of the right coronary artery was crossed using a Safe-Cross radio frequency guidewire and the procedure was completed through implantation of rapamycin-coated stents. Excellent results were seen in both treated lesions by angiography at 6 months. The results obtained during follow-up would have been unthinkable 20 years earlier using percutaneous revascularization techniques.
Although for a number of years surgery has been the preferred option for main artery disease in the majority of centers,75,76 percutaneous revascularization with stents has been practiced in parallel since 1991. Originally, patients were treated by necessity in the catheterization laboratory as a result of a complication during diagnosis or in the course of myocardial infarct. Cardiopulmonary support was always useful in critically ill patients.77 Later on, percutaneous treatment started to be used in patients in whom coronary bypass surgery had been rejected78 due to concomitant systemic disease, poor distal beds, or poor ventricular function. Although the initial primary success rate was high, the rates of restenosis represented one of the limitations of percutaneous interventional cardiology, with a reported incidence of close to 20%.79-83 The first studies with rapamycin-coated stents for the treatment of left main coronary disease have shown promising results,84 with a restenosis rate of 3%. As recently suggested in this journal,85 perhaps these results indicate that it is now time to change the guidelines.
FUTURE PERSPECTIVES
Despite the incorporation of all of the improvements that have arisen in recent years, the techniques continue to progress and it is possible that solutions will arise in the near future to the current problems associated with percutaneous interventional cardiology. The navigability and accessibility of current stents could be improved to approach that of the most recently developed balloons, allowing them to access distal lesions with multiple proximal tortuosities. The Safe-Cross radio frequency guidewire has opened new roads into the resolution of chronic occlusions. New models of this device will be more flexible, display improved navigability, be able to move around curves without losing penetration, and, at the same time, achieve greater safety.
Studies have begun in which new drugs are being investigated for use in drug-coated stents that could offer improvements on those currently available in terms of restenosis and long-term results. Absorbable stents are now also in an advanced stage of development and it is possible to consider a combination of all of these technologies. The advances will not only apply to the treatment of coronary lesions, but also to the field of ventricular function. Often, following successful treatment of coronary lesions, a degree of ventricular dysfunction can persist that severely limits functional capacity. Myocardial regeneration using percutaneously injected stem cells, either by intracoronary catheterization or endomyocardial injection, is another great chapter recently opened in the field of interventional cardiology. Consequently, it is possible that in the not too distant future a substantial improvement in the survival of patients with coronary disease will be achieved and we will have to address other forms of atherosclerosis in other locations that are currently less effectively resolved.
Section Sponsored by Laboratorio Dr Esteve
Correspondence: Dr. M. Pan.
Grupo CORPAL. Hospital de la Cruz Roja.
P.o de la Victoria, s/n. 1. 14004 Córdoba. España.
E-mail: grupo_corpal@arrakis.es.