Keywords
INTRODUCTION
The outcome of percutaneous coronary interventions depends on a number of clinical, anatomical, and technical factors that are often overlapping and interdependent. The predicted results of coronary angioplasty have been established since 19861 in the classic risk stratification of the American College of Cardiology/American Heart Association (ACC/AHA). This classification, currently in widespread use, has various limitations, such as the interobserver variability in the angiographic classification of the lesions and the fact that neither ventricular function nor certain clinical characteristics of the patient or symptom presentation are taken into consideration. Furthermore, changes in the techniques used, greater experience, and the universal application of the stent, along with the recent availability of drug-eluting stents, have led to a substantial change in the current focus of interventional cardiology.
Attempts have been made to achieve a more precise definition of the risks of interventional cardiology. Among these, a group from the Mayo Clinic established a score based on 5 clinical and 3 angiographic variables (Figure 1).2 Compared with the ACC/AHA classification, this score shows a greater discriminatory capacity for establishing the risk of major complications following angioplasty, true determinants of long-term prognosis; nevertheless, the ACC/AHA classification is still slightly better at predicting immediate angiographic failure. This review will address the influence of various clinical and anatomical factors on the current results of percutaneous coronary interventions.
Figure 1. Prediction of postintervention complications according to the Mayo Clinic score. Clinical and angiographic variables with their corresponding score shown above a graph of the 5 risk categories for major complications. NYHA indicates New York Heart Association; PTCA, percutaneous transluminal coronary angioplasty.
STABLE ANGINA
Patients with stable angina generally have milder associated coronary pathology and preserved ventricular function. Consequently, the risk of significant clinical events is not high. In classic studies in which medical therapy was compared with balloon angioplasty,3,4 no significant differences were observed in terms of mortality or infarct, although better exercise tolerance and a lower incidence of angina was found in patients who had received angioplasty, but with a higher rate of surgery for restenosis.
The use of stents has caused a notable reduction in restenosis, leading to a change in the protocols for patients with stable angina. Not excluding correct treatment with aspirin, antianginal drugs, beta-blockers, statins, and health checkups, angioplasty with stent implantation is the treatment of choice for the large majority of lesions, with the exception of, particularly, lesions of the left main coronary artery. An interesting study is underway using data from long-term follow-up (5 years), the COURAGE trial,5 in which angioplasty and intensive medical treatment are compared with isolated aggressive medical management in patients with 1 to 3 diseased vessels; the results will provide an evaluation of current procedures.
UNSTABLE ANGINA
Pharmacological treatment of patients with acute coronary syndrome without ST elevation includes, in addition to standard antianginal therapy, administration of antiplatelet drugs upon hospital admission,6-8 including aspirin, clopidogrel, and glycoprotein IIb/IIIa inhibitors, and the simultaneous use of antithrombotics such as low-molecular-weight heparin.9
The first question that must be addressed in the management of patients with acute coronary syndrome without ST elevation is which patients will benefit from an initial strategy of percutaneous intervention. Identification of these patients requires individual risk stratification that has a high predictive value and can be applied almost immediately on admission.10 A second question arises if an initial interventional strategy is adopted, and consists of determining when the procedure should be performed or the optimal period of time before performing coronary angiography.11
In recent years, sufficient data have appeared to support the use of an initial invasive strategy, especially in intermediate or high-risk patients, over passivation or "cooling off" of the plaque.12-14 All these studies had in common that invasive treatment was performed, on average, in the first 24-72 hours and that intensive antithrombotic and antiplatelet treatment had been initiated following diagnosis. A recent study, ISAR-COOL, places even greater emphasis on the optimal timing of the intervention.15 A group of 410 patients with unstable angina and ST-segment depression or elevated levels of troponin T was randomized for early percutaneous intervention within 6 hours (mean of 2.4 hours) or for a strategy of "cooling off" for 3 to 5 days prior to the intervention (mean of 86 hours). From admission, all patients received aspirin, clopidogrel, tirofiban, and intravenous unfractionated heparin. Death or infarct at 30-day follow-up was significantly reduced in the group that received early treatment (5.9% vs 11.6%; P=.04). This difference was mainly due to a higher number of infarcts prior to intervention in the group subjected to a "cool off" strategy.
An alternative to standard intravenous antithrombotic and antiplatelet treatment concomitant with percutaneous intervention is shown in the results of the REPLACE II study,16 which demonstrates the safety and efficacy of a new antithrombotic, bivalirudin, compared with a combination of heparin and abciximab as a unique treatment during the percutaneous interventional procedure. The results of studies such as the ACUITY study17 that aim to define the role of bivalirudin in high-risk patients with unstable angina who receive early treatment will be released during the coming year.
Current guidelines18 for low-risk patients with acute coronary syndrome without ST elevation indicate ischemia-guided coronary angiography. In intermediate or high-risk patients (especially those showing electrocardiographic changes or elevated levels of enzyme markers) an early intervention strategy is recommended; coronary angiography within the first 24 to 48 hours appears to be sufficient given the logistic variability inherent in the Spanish health care system.19
RENAL FAILURE
The incidence of contrast nephropathy in patients who have undergone coronary angiography or a percutaneous revascularization procedure can vary according to the definition criteria used.20 If we use an increase in the level of serum creatinine of >25% of basal levels, the incidence varies from <1% in healthy individuals to up to 50% in high-risk patient groups. The presence of renal failure worsens the prognosis21 and can even double mortality in patients who receive percutaneous coronary interventions. Although the etiology is multifactorial, the intense medullary vasoconstriction induced by the contrast agent is the most common cause of nephropathy and patients with prior renal dysfunction present a higher risk, especially those with diabetic nephropathy. Other risk factors in these patients include the use of large volumes of contrast agent, hypovolemia, and left ventricular dysfunction.
The main means of protection, especially in high-risk patients, is adequate hydration by administration of isotonic saline (100-150 mL/h) for 8 to 12 hours prior to the procedure and for the following 12 to 24 hours.22 The use of nonionic isosmolar contrast agents (iodixanol)23 instead of low osmolarity agents (iohexol) and, in particular, the quantity used, can affect the severity of the renal damage.
Prior treatment with N-acetylcysteine (600 mg every 12 hours the day before and the day after the procedure) appears to reduce the incidence of nephropathy in patients with moderate renal failure, especially when it is used alongside appropriate hydration and the use of small amounts of contrast agent.24 Administration of N-acetylcysteine as an intravenous bolus 4 hours prior to the procedure can be beneficial when it is necessary to perform urgent procedures.25
The use of fenoldopam, a dopaminergic agonist, prior to the procedure does not appear to have any protective effect against nephrotoxicity. A recent study comparing N-acetylcysteine with fenoldopam, along with correct hydration, showed a clear benefit with N-acetylcysteine when comparing the percentage of patients showing increases of at least 0.5 mg/dL serum creatinine (4.1% vs 13.7%; P=.019).26
It has been suggested that the use of postprocedural hemofiltration is beneficial in patients with increased creatinine levels (>2 mg/dL). Thus, a recent study showed a significant reduction in the rate of in-hospital events (52% vs 9%; P<.001) and a reduction of in-hospital mortality from 14% to 2% (P=.02) in patients who received hemofiltration.27
DIABETES MELLITUS
More than half of all adult diabetics have significant coronary atherosclerosis, with a prevalence 10 times higher than seen in the general population (around 2%-4%).28 Diabetic patients represent 15%-25% of all patients who receive percutaneous coronary interventions and the short and long-term results are worse than in nondiabetic individuals,29 essentially due to the presence of more diffuse vascular disease with smaller vessels, a high degree of thrombogenicity, and a higher level of intimal hyperplasia, all of which translates into a higher rate of restenosis following angioplasty.
There are 3 ways in which to improve the results of interventional cardiology in diabetic patients: optimize the pharmacological treatment of coronary syndromes, improve the interventional devices, and improve secondary prevention.
Diabetic patients have received the greatest benefit from treatment during intervention with glycoprotein IIb/IIIa inhibitors, especially abciximab and tirofiban. The beneficial effect is observed both in stable situations and in acute coronary syndromes,30,31 with a reduction in mortality at 30-day follow-up of 70% and at 1-year follow-up of 45%.
The metallic stent has improved initial and long-term results, and has reduced the rate of restenosis compared with balloon angioplasty. However, these results are not applicable to diabetic patients, especially those suffering from type 1 disease.32 The development of stents that elute inhibitors of intimal hyperplasia has represented a huge step forward in the reduction of in-stent restenosis. There are currently 2 drugs available, rapamycin and paclitaxel, and available data from randomized studies indicate an 80% reduction in the rate of angiographic restenosis in diabetic patients at 9-month follow-up.33,34 In turn, the requirement for repeat revascularization ranges, according to the study, between 2%-7% and 20%-56% (P<.001) in patients treated with drug-eluting and conventional stents,
respectively. In a recent study by Sabaté et al35 undertaken in a group of diabetic patients randomized to receive either rapamycin-eluting or conventional stents, a reduction in the rate of restenosis (5% vs 31%; P<.0001) and in the requirement for repeat revascularization (7.5% vs 31%; P<.0001) was observed.
Independently of the drugs and devices used, it is particularly important to maintain careful control over glucose levels following the procedure, as well as recognizing "prediabetic" states, in which slight increases in fasting glucose level or of hemoglobin A1c are associated with increased risk of death, restenosis, and requirement for repeat revascularization.36,37
MULTIVESSEL DISEASE AND VENTRICULAR FUNCTION
The classic data from the CASS study established that revascularization is indicated in patients diagnosed with multivessel disease and normal ventricular function to improve symptoms and functional capacity; if ventricular function is diminished, the objectives will include prolonged survival.
To determine whether the best revascularization strategy is represented by angioplasty or coronary surgery, numerous randomized studies were undertaken in which more than 5000 patients were included.38 The majority of these studies, however, were undertaken when stents and glycoprotein IIb/IIIa inhibitors were not used systematically. Consequently, although the results at 1 to 3-year follow-up were similar in terms of death and/or infarct, a tendency toward greater long-term benefit was seen with surgery, especially in terms of the need for repeat revascularization. This benefit was even more apparent in diabetic patients.
However, 2 important issues were brought to light: a) patients referred for one technique or the other are different, presenting difficulties for the design and interpretation of clinical studies; and b) restenosis, the factor which best explains the differences in the results, has continued to reduce since the systematic use of the stent.
The inclusion and exclusion criteria were very strict and varied according to the study, in such a way that, overall, less than 10% of patients considered eligible were finally randomized, suggesting that the results might not be applicable to all patients with multivessel disease. An important issue affecting long-term results is not so much the mode of revascularization as whether or not it is complete, either from an anatomical perspective (revascularization of all stenoses of >50% in vessels ≥2.0 mm) or a functional one (revascularization of only those lesions that cause ischemia). Data from the EAST study39 suggested that when functionally equivalent revascularization is obtained with angioplasty and surgery the long-term results will be the same. Likewise, when function is diminished, especially in diabetic patients, anatomically complete revascularization is justified, while if function is normal, a functionally complete revascularization may be sufficient.
The use of potent antiplatelet drugs and the generalized use of the stent has led to an increase in the complexity of the lesions and patients treated with multivessel angioplasty, without any apparent worsening of immediate results in terms of in-hospital mortality and/or infarct and, if anything, an improvement.40 In patients with multivessel disease who have a high risk for surgery, percutaneous intervention using stents is an effective alternative in the medium term, with rates of immediate in-hospital complications lower than with the surgical option, as shown by the data published from the AWESOME study.41 In the long term, data is available from various randomized studies comparing surgery with angioplasty with stents (ARTS 1 and ERACI II)42,43; all of them show, at 2 to 3-year follow-up, survival without infarct or cerebrovascular accident that is similar in the 2 strategies, but with a requirement for repeat revascularization that, although clearly lower than found in previous studies using balloon angioplasty, were still higher in the group who received percutaneous treatment.
The good results obtained using drug-eluting stents33,34 once again casts doubt upon the practical use of the data obtained to date. Preliminary data is now available from the ARTS 2 study,44 in which data obtained in the ARTS 1 study was compared with results obtained in a group of patients with multivessel disease, but with a worse clinical profile, treated with drug-eluting stents. The rate of complications at 1-month follow-up in patients in the ARTS 2 study was 2.8%, compared with 4.1% and 8.2% in the groups who underwent surgery or percutaneous intervention, respectively, in the ARTS 1 study. In turn, the rate of major events at 6-month follow-up was 5.3% in ARTS 2 compared with 9.0% and 20% in the groups that received surgery and percutaneous intervention, respectively, in ARTS 1, due to a significant reduction in the requirement for repeat revascularization as a result of restenosis. While awaiting the results at 1-year follow-up, it appears that with drug-eluting stents we are close to achieving similar long-term results to those obtained with surgery (Figure 2).
Figure 2. Event-free survival in 2 randomized studies comparing surgery and angioplasty in patients with multivessel disease (CABRI and ARTS 1) and predicted data from the ARTS 2 study for drug-eluting stents. The long-term results with drug-eluting stents equal the best surgical results. AMI indicates acute myocardial infarct; RS, revascularization surgery; PTCA, percutaneous transluminal coronary angioplasty.
LOCATION OF THE LESION
Saphenous Vein Grafts
Angioplasty of saphenous vein grafts is a problem that remains to be fully resolved in interventional cardiology and shows a high rate of immediate complications and poor long-term results. Immediate or subacute complications are associated with distal embolism and reduced anterograde flow (the no-reflow phenomenon), while in the long term the problem is increased restenosis.
Distal embolization of part of the plaque, which causes acute occlusion of distal portions of the native vessel, occurs in 2-15% of cases. Although some results are available that indicate the usefulness of glycoprotein IIb/IIIa inhibitors, other observational studies indicate a limited benefit associated with this strategy.45 In recent years, various mechanical systems have been developed that prevent distal embolization and various studies have been published that demonstrate their efficacy, due essentially to a lower incidence of myocardial infarct (8.6% vs 14.7%) and a reduction of the no-reflow phenomenon (3% vs 9%).46,47 When the clinical symptoms and angiographic appearance suggest the presence of recent thrombotic material, in addition to the systems mentioned, thrombectomy devices can also be employed. Apart from observational studies, there are currently only 2 randomized studies48,49 available that, although with disparate results in terms of overall clinical benefit, suggest that these procedures reduce the extent of postprocedural myocardial infarcts.
The reduction in anterograde flow, also known as the no-reflow phenomenon, has an uncertain etiology but may be due to microembolizations and/or microvascular spasm, and its frequency can reach up to 12% of procedures. Although protection systems can prevent this phenomenon, its treatment is different and various drugs can be effective by intracoronary administration. Such drugs include vasodilators such as nitrates, nitroprusside, or adenosine, and calcium antagonists such as verapamil.
The high rate of restenosis following balloon angioplasty led to stent implantation being clearly indicated in percutaneous treatment of saphenous vein grafts.50 Nevertheless, the long-term results were never as good as those obtained in native arteries and displayed a possibility of restenosis that extended beyond the first 7 months, with a risk that continued up to 18 to 24 months due to the special progressive nature of the disease in regions in which a stent had initially been used.51 Although the majority of available randomized studies of drug-eluting stents exclude patients with saphenous vein grafts, they have been included in recent register studies52 in which the consecutive activity was collected, including all types of lesions, for various interventional cardiology teams, with excellent medium-term results and rates of repeat revascularization for restenosis of <5%.
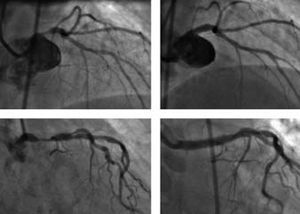
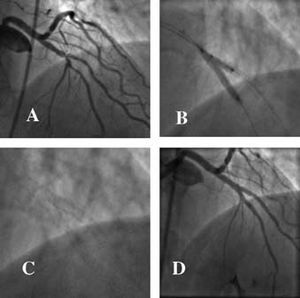
Left Main Coronary Artery
Left main coronary artery disease is present in 3%-5% of patients in whom coronary angiography is performed. Although it has always been considered as a standard indication for surgical revascularization,53 treatment of lesions of the left main coronary artery is technically feasible and, since the introduction of the stent, many groups have reported their results. These results depend upon various factors: a) the clinical conditions in which the procedure is undertaken (elective or emergency); b) the presence of protection via a venous or arterial graft; and c) the degree of ventricular function.
In a current series in which all types of patient have been included, in-hospital mortality rates of 15% were obtained (3.7% in elective cases and 45.5% in urgent cases).54 The cumulative probability of survival for the elective group was 86% at 3-year follow-up, compared with 54% from 6 months onwards in the urgent group. A similar study showed an overall survival at 1-year follow-up of 88%, which increased to 95% in protected arteries and was reduced to 72% in unprotected arteries; the rate of major adverse clinical events was 25% and 49% for the protected and unprotected groups, respectively, and repeat revascularization for restenosis was required in 20% of patients with no differences between the groups.55 A series with longer term results in which only unprotected arteries in elective procedures were included showed a survival rate at 3-year follow-up of 91%, with a cardiac mortality of 11.9% and a restenosis rate of 31%.56
The limiting factor imposed by the high rate of in-stent restenosis could be resolved, in part, by the availability of drug-eluting stents. Although experience is still limited, some series have begun to appear that show very good immediate results and restenosis rates at 1-year follow-up of 4%-8%, with a requirement for repeat revascularization in 2%-4% of patients and a mortality of no more than 1%.57-59
Nevertheless, the current guidelines for interventional cardiology60 that establish surgical treatment of the left main coronary artery as a class I indication continue to be applicable; likewise, angioplasty is a class IIa indication in favorable lesions with a protected artery or, if it is unprotected, when the risk of surgery is high, and a class IIb indication in unprotected arteries (Figure 3).
Figure 3. Two cases of elective angioplasty in an unprotected left main coronary artery. A) Noncalcified ostial lesion in a 57-year-old clinically stable woman with a high probability of angiographic success (class IIb indication). B) Result following implantation of a drug-eluting stent. C) Lesion in the middle third of the artery of an 85-year-old woman with high surgical risk (class IIa indication). D) Result following implantation of a drug-eluting stent.
Ostial Lesions
Ostial lesions can be defined as those that are located within 3 mm of the ostium of the anterior descending, the circumflex, or the right coronary artery. Classically, these lesions were associated with an inadequate angiographic result following balloon angioplasty due to high rigidity and a large elastic component. Various atherectomy devices, using cutting or abrasion,61 or cutting balloon angioplasty,62 were effective in obtaining a better immediate angiographic result but presented a high restenosis rate (40%-60%).
The stent, used alone or following atherectomy, has reduced the risk of elastic recoil and severe dissection, leading to a good immediate result and lower rates of restenosis (20%-35%).63 A recent retrospective study compared the outcome at 1-year follow-up of stenting in ostial lesions and proximal, nonostial lesions.64 While the immediate results were similar (93% vs 97%), event-free survival at 1-year follow-up was lower in the ostial lesions (69% vs 80%; P<.002), mainly due to a greater requirement for repeat angioplasty (19% vs 10%; P<.0001). Although there are no studies specifically addressing the use of drug-eluting stents in this type of lesion, data obtained on the consecutive activity of a single center in which drug-eluting stents were used exclusively showed significantly lower rates of restenosis than seen with conventional stents.65
Bifurcation Lesions
A bifurcation lesion can be defined as presenting at least 70% stenosis of the main vessel and also of the ostium of the secondary branch or side branch. According to this definition, up to 15% of all percutaneous interventional procedures are performed in bifurcation lesions. Traditionally, balloon angioplasty was associated with a low success rate and a relatively high incidence of immediate complications,66 often due to problems related to dissection or occlusion of the side branch. Techniques using double balloons or atherectomy did not produced the hoped for results, either immediate, with a higher number of in-hospital complications, or long term, with a high rate of restenosis. Use of the stent initially succeeded in reducing acute complications and lowering the rate of restenosis, principally that associated with the main branch.
Before considering the different techniques with which to approach bifurcation lesions using stents, the first question to be addressed is whether initial treatment will focus on the main artery or whether combined treatment of both vessels using stents will be considered from the outset. For this, it is necessary to establish the importance of the side branch, either in terms of its diameter or, in particular, the extent of the myocardium that it supplies.
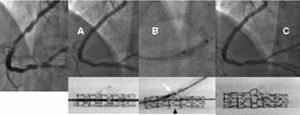
Various stent implantation techniques have appeared in recent years. The French group led by Lefevre67 established 4 main options according to the order and form of stent colocation in the main branch and side branch.
If the stent is to be implanted in both branches, T stenting or the more recent technique of "crushing" is used. The latter technique begins with the simultaneous positioning of 2 stents in the 2 branches of the bifurcation. Next, the stent in the side branch is expanded such that it extends 3-5 mm into the lumen of the main branch. Subsequently, the main stent is expanded so that the portion of the stent in the side branch that extends from the ostium is literally crushed against the arterial wall. It is advisable to complete the procedure with the simultaneous inflation of balloons in both branches in order to improve access to the side branch. The use of this method ensures complete metallic coverage of the ostium of the side branch at the expense of implanting 3 overlapping metallic layers in a small area of the main vessel (Figure 4).
Figure 4. Severe lesion in the anterior descending/diagonal artery. Crushing technique. A) Initial lesion. B) Kissing balloon following implantation of the stents in the 2 arteries. C) Image of the 2 stents shown without contrast agent allowing visualization of the complete coverage of the ostium of the diagonal artery. D) Final angiographic image.
If, on the other hand, a single stent is initially implanted in the main vessel, the technique used should allow a second stent to be passed through the first and inserted into the side branch should such a procedure be justified on clinical or angiographic grounds. This concept is known as provisional stenting (Figure 5).
Figure 5. Severe lesion in the distal bifurcation of the right coronary artery. A) Implantation of a drug-eluting stent towards the posterior interventty through the mesh of the stent towar posterolateral artery. C) Good angiographic result, making stent implantation in this branch unnecessary. The lower panels show in vitro examples of the technique described.
Although the immediate angiographic results using the techniques of T stenting and provisional stenting are excellent (92%-98% success),68,69 with a rate of immediate complications of 2%-5%, the longer term results show rates of angiographic restenosis that remain high (17%-53%), particularly in the side branch and generally at its origin. The reason for this poor evolution is, in many cases, the impossibility of covering the ostium correctly with the stent due to the severe angulation at the origin of the side branches.
A number of groups have now presented their results with drug-eluting stents70,71 using a variety of techniques for the implantation of 1 or 2 stents. The restenosis rates are between 3% and 9% for the main vessel and between 13% and 25% for the side branch. The rate of restenosis of the side branch was lower (7%) in cases employing crushing (a technique that ensures a better coverage of the ostium) and also in cases in which provisional stenting was used (14%). An alternative to these techniques is the use of so-called bifurcated stents. These have recently become available and are not yet produced as drug-eluting stents, and although they are currently insufficiently tested, they may offer improved access to the side branch.
Calcified Lesions
Calcification is an important aspect of atherosclerotic plaque pathology. It appears to varying degrees depending upon the composition and age of the plaque, and its extent is as important as the distribution in the plaque and the arterial wall. Although these characteristics are best measured by intravascular ultrasound,72 they are normally evaluated radiographically during angiography; however, the sensitivity of the method for the measurement of small or moderate amounts of calcium is low and its ability to determine the extent of calcification is only moderate.
Calcification is not only important in the lesion to be treated but also in the arterial wall. An artery that is calcified along its length can cause difficulties in navigating the balloon or other devices, complicating the arrival of the stent at the lesion site. In addition, calcium in the lesion can impede correct expansion of the balloon, despite the use of high inflation pressures. Although the presence of calcium is not, by itself, a factor associated with increased risk of restenosis, arterial rigidity impedes the achievement of an adequate lumen diameter, which shows an inverse relationship with the development of restenosis. Thus, it is important to obtain the largest lumen diameter possible. To this end, the stent is implanted using high inflation pressures that ensure correct expansion and placement of the stent against the arterial wall.
If appropriate balloons cannot be symmetrically inflated using high pressures, rotational atherectomy (Rotablator) is used. This fragments part of the calcium into microparticles such that arterial rigidity is overcome and it is possible to perform subsequent inflations to allow stent placement. Available data on the use of this technique show an immediate angiographic success of >95% and a rate of in-hospital events of 3.5%.73 When the procedure was finalized with implantation of a stent, the requirement for repeat revascularization due to restenosis was 15%.
An alternative would be the use of a cutting balloon,74 which, although it would be more indicated in fibrous lesions, can fragment, via longitudinal cuts, the superficial calcification of the plaque to facilitate improved distensibility of the lesion and allow correct dilatation and expansion of the stent.
It is reasonable to hope that the same results obtained with drug-eluting stents in less complex lesions will also be achieved with calcified lesions; the results obtained from a series including all types of lesion but with a high percentage of calcified lesions show excellent medium and long-term clinical outcome.75
Chronic Occlusions
Angioplasty in chronically occluded lesions represents 10%-15% of routine percutaneous interventional procedures. Chronic occlusions are principally made up of calcified plaques and it is difficult, or sometimes impossible, to cross them with devices specifically designed for the purpose. This leads to a low success rate, high material costs, increased radiation dose, and above all, a higher frequency of restenosis and reocclusion in the medium term compared with nonocclusive stenoses.
A review of the literature, which contains more than 4400 treated chronic occlusions, reveals an angiographic success rate of 69% (47%-81%). The most common causes of failure were as follows: impossibility of crossing the lesion with the guidewire (80%), failure of the balloon to cross the lesion (15%), and inability of the balloon to dilate the stenosis (5%). For situations in which standard guidewires consistently fail, a special system has been developed in which the guidewire uses optical coherence reflectometry to differentiate between the vessel wall and the lumen, thereby avoiding the risk of perforation. At the same time, the system can emit radiofrequency pulses that facilitate passage through the fibrotic material. Data from a study using this device76 demonstrate its efficacy in obtaining angiographic success in more that half of the procedures that previously failed using standard guidewires; however, it should be stressed that the number of perforations was not insignificant (2.6%).
Many studies have been published on the advantages of using stents in this context. Nevertheless, the rates of angiographic restenosis range from 32% to 55% and repeat revascularization is required in 15%-25% of cases. There are various reasons for this poor long-term outcome. Frequently, these lesions are calcified, located in ostial or bifurcated sites, and in addition, are usually long (>15 mm), factors which independently favor an increased probability of restenosis.
Available data suggest that one of the clearest applications of drug-eluting stents is their use in chronic occlusions. Data published by the Rotterdam group77 on 56 consecutive patients with chronic occlusions treated with drug-eluting stents shows a rate of angiographic restenosis at 6-month follow-up of 9%. At 1-year follow-up, the percentage event-free survival (without infarct, death, or repeat angioplasty) was 96.4%. A prospective, nonrandomized study (the SICTO study) has just released preliminary results on 25 patients treated with drug-eluting stents.78 At 6-month follow-up, only 2 patients required repeat angioplasty in lesions that were not related to the implanted stents.
Small Vessels
One third of all angioplasties are performed in arteries with a diameter of <3 mm, a factor that has an important effect on the outcome of the procedure. In addition, small-diameter arteries are associated with other factors such as diabetes and diffuse atheromatosis that contribute further to poor long-term prognosis. The use of the stent has improved results but restenosis rates are still obtained that are higher than with vessels of a larger diameter.
The main cause of in-stent restenosis is neointimal proliferation. For the same amount of neointimal proliferation, the reduction of lumen diameter will logic ally be greater the smaller the diameter of the stent used. This is why the rate of restenosis is higher in small-diameter vessels.
Numerous studies have compared stent implantation with balloon angioplasty in small vessels, although some have obtained conflicting results. In a recent metaanalysis in which data was collected from 11 randomized trials, an overall rate of angiographic restenosis of 25.8% was obtained with stent implantation compared with 34.2% with balloon angioplasty (P=.003).79 While there were no differences in mortality or the incidence of myocardial infarct, the requirement for repeat revascularization was lower in the stent implantation group (12.5% vs 17%; P=.004) (Figure 6).
Figure 6. Comparison of studies performed in small diameter arteries in terms of late lumen loss (reduction of the immediate gain in lumen diameter) in the angiographic follow-up. Reference diameter is indicated in mm alongside the study acronym. The 3 studies that used drug-eluting stents, Sirus, Ses-Smart, and Research, display a late lumen loss that is clearly less than with conventional stents.
A subanalysis of the main studies published using drug-coated stents33,34 reveals a benefit that also extends to patients with arteries between 2.3 mm and 2.8 mm in diameter, with restenosis rates that vary between 16% and 6%, respectively, and a reduction in risk of between 57% and 83%. In the SES-SMART study80 patients with arteries <2.75 mm were randomized to receive either rapamycin-eluting or conventional stents. The restenosis rate was 9.8% vs 53% with a requirement for repeat revascularization of 7% vs 19.3% in the groups receiving rapamycin-eluting and conventional stents, respectively. A subanalysis of the RESEARCH registry of the use of drug-eluting stents from the Rotterdam group assessing treatment with rapamycin-eluting stents in very small vessels (1.88±0.34 mm) shows excellent results, with an angiographic restenosis rate of 10% and a requirement for repeat angioplasty at 1-year follow-up of 5.5%.81
Correspondence: Dr. J. Zueco Gil.
Unidad de Cardiología Intervencionista. Hospital Universitario Marqués de Valdecilla.
Avda. Valdecilla, s/n. 39008 Santander. Cantabria. España.
E-mail: hemodinamica@humv.es