Keywords
Acute myocardial infarction (AMI) is a syndrome characterized by acute disruption of the coronary blood flow. This is due to a combination of rupture of atherosclerotic plaque secondary to several causes, and the formation of a thrombus which blocks the vessel. There are other interrelated factors, such as spontaneous thrombolysis, vasoconstriction, the presence of collateral circulation, etc, that also play an important role in the development of this syndrome. The final extent of myocardial necrosis depends on endogenous mechanisms and, above all, on the treatment implemented.1 In this update we review current concepts on percutaneous revascularization in AMI.
PRIMARY PTCA. FACILITATED PTCA (WITH OR WITHOUT TRANSFER TO ANOTHER CENTER)
Comparison With Thrombolysis
Percutaneous transluminal coronary angioplasty (PTCA) was introduced as a reperfusion strategy in the mid-1980s. Primary PTCA (in the context of AMI) was described by Meyer et al2 and Hartzler et al3 in 1982-1983. This technique proved to be superior to thrombolytic therapy regarding early and complete restoration of coronary blood flow,4 with a low incidence of recurrent ischemia, reinfarction, stroke, and death.5-7 In the STAT8 study, primary PTCA was compared with thrombolysis (TBL).
In the first group, the primary end point (death, reinfarction or stroke) was reduced significantly (P<.001). In a study conducted in our center in the 1990s, where primary PTCA was compared to thrombolysis via accelerated t-PA in patients with anterior AMI, significant benefits were found regarding mortality, residual ischemia, reduction in cardiac rupture, and stroke.9 This benefit was maintained at 4-year follow-up in patients >65 years old. Weaver et al10 analyzed the results of the first ten randomized studies which compared primary angioplasty with thrombolysis in a metaanalysis that demonstrated lower mortality, reinfarction, and stroke in the patients treated with primary PTCA.10
Zijlstra11 reviewed 6478 patients randomized to receive primary PTCA versus TBL. Mortality was 5.5% in the first group and 7.8% (P<.001) in the second. Keeley et al12 reviewed all the studies which compared PTCA with thrombolysis in AMI, totaling 7739 patients. The patients treated with PTCA had lower mortality (P=.0002), non-fatal reinfarction (P<.0001), and a smaller number of adverse events. These results were better in the short-term and at follow-up independently of the thrombolytic used. In the TBL group, bleeding was significantly greater than in the patients treated with PTCA (P<.0001). The NHMRC group presented the results of different studies on patients at 6-month follow-up where primary PTCA was compared with thrombolysis.13 In the PTCA group 30-day mortality was 4.3% and 6.9% (P=.004) in the TBL group. The difference was still significant at 6 months (P=.04).
Relationship of Delay to Effectiveness
Reperfusion therapy should begin as early as possible: "time is muscle." In each case the dilemma between delay in establishing treatment and its effectiveness will influence the choice of the best therapeutic strategy in each case.
Zijlstra et al14 evaluated patient evolution according to the time of establishing treatment. They studied 2635 patients enrolled in ten randomized studies of primary PTCA versus thrombolysis. The mean time from randomization until the treatment was 69 min for PTCA and 22 min for TBL. The patients were divided into 3 groups depending on whether they were randomized <2 h from the onset of pain between 2 and 4 or 4 h.
The patients treated with PTCA had the lowest incidence of major adverse events, regardless of the time from the onset of pain to the time of randomization. As time increased the number of events increased in the thrombolysis group, but not in the PTCA group. In the PAMI 2 study,15 mortality was lower in the patients treated before 2 h, but was independent of time after this. Although we can also speak about the "golden hour" in PTCA,16 which should better preserve ventricular function,17 delay has a stronger effect on TBL: the efficacy of thrombolytic treatment is less and the relative benefit of PTCA is still greater after the first hour. Brodie et al17 evaluated the importance of the time of reperfusion on 30-day survival and left ventricle ejection fraction (LVEF).
The 1352 patients analyzed were divided into 2 groups depending on the onset of pain, i.e., more than or less than 2 h before treatment. In the first group, early mortality was less (P=.04). In the patients treated after >2 h, mortality at 30-day and late mortality were independent of length of delay.
Brodie et al analyzed the time of reperfusion of patients included in the Stent PAMI study.18 They divided the patients according to time of randomization; i.e., <2 h from pain onset 2 to 4 6 and 6 h. There was a trend toward presenting a higher number of anterior infarctions in the first group of patients. No differences in mortality were found at 1 month and 6 months regarding new target vessel revascularization (TVR) due to ischemia or disabling stroke.
At 6 months there were a higher number of reinfarctions in the late presentation group. Similar TIMI grade 3 flow was found in all groups. However, LVEF was higher in the patients treated before 2 h. Reocclusion was greater in the late revascularization group without this being influenced by the use of stenting. It has been verified that primary PTCA achieves better TIMI grade 3 flow, reduces mortality due to mechanical complications, and decreases hemorrhagic complications. The greatest benefit obtained with primary PTCA compared to thrombolysis is partly due to the stronger impact of delays on thrombolysis.
Risk Assessment in PTCA
Addala et al19 presented a mortality risk score in patients with AMI treated with PTCA. Total mortality was 3.1%. The study demonstrated scores strongly associated with hospital mortality at 1 month and 6 months. This increased 33 times if the score was ≥9 (P<.0001). The predictor with the greatest weight was age >75 years which reached a score of 7. The most important finding in this study is that it defines a group of patients who should receive more aggressive treatment. Primary balloon angioplasty was safe and more effective than thrombolysis. However, during angiographic follow-up reocclusion of the target vessel was found in 10%-15% of cases and restenosis in 35%-40%.20 The presence of residual dissection or stenosis >30% were the most important predictors of recurrent ischemia and reocclusion of the target vessel.21
Balloon Angioplasty or Coronary Stenting?
The limitations of primary balloon angioplasty led some researchers to include stents in the therapeutic arsenal for treating AMI. At first infarction was considered a contraindication for stenting due to the theoretical risk of occlusion when implanting it in lesions with high thrombotic content.22 However, obtaining greater luminal diameter with the stent and resolving the residual dissection (predisposing factors for ischemia and arterial reocclusion)23 could facilitate the resolution of the residual thrombus by endogenous fibrinolytic mechanisms. Numerous published studies and registries have demonstrated the safety and efficacy of stenting during primary PTCA.
Stone et al24 presented the results of the PAMI Stent Pilot Trial where the safety of stenting in AMI was evaluated. The patients had a low incidence of hospital death (0.8%), reinfarction (1.7%), and recurrent ischemia (3.8%). In a later publication, the results of 7-month follow-up25 were presented with data from 236 patients. Mortality was 1.7%, reinfarction 2.1%, and TVR 11.1%.
Angiographic restenosis was 27.5%. The number of stents and the vessel reference diameter were determinants of TVR. Grines et al26 published a study which compared PTCA with and without stenting. A total of 900 patients were included. A greater minimum lumen diameter (MLD) was obtained in the group treated with stenting (P<.001). At 6 months, the patients in the stent group had the lowest incidence of angina (P=.02), restenosis, and ischemia-driven TVR (P<.001). The combined primary end point of death, reinfarction, disabling stoke, and TVR was also significantly smaller. However, in the group treated with stenting a trend was observed toward greater mortality at 12 months, although it did not reach significance. This coincided with a smaller degree of TIMI grade 3 flow in the stent group, probably due to distal embolization of the thrombus previously fragmented by predilatation with the balloon. The STENTIM 1 study demonstrated the safety of stenting in AMI.27
The PASTA study28 demonstrated that primary PTCA with stenting in selected patients had a low incidence of major cardiac events during the first 12 months and lower rates of restenosis compared to balloon angioplasty.
Other additional studies have helped to demonstrate the safety and efficacy of stenting in treating AMI, such as those carried out in our centre,29 the STENTIM-2 study,30 and the FRESCO study.31 The CADILLAC study32 took a further step and included glycoprotein IIb/IIIa inhibitors; PTCA with stenting (Multilink) was compared to balloon angioplasty in patients either receiving or not receiving abciximab. A total of 2082 patients were included and divided into: primary PTCA (n=515), primary PTCA with abciximab (n=529), stenting (n=513), and stenting with abciximab (n=525).
The primary end point was the combination of death, reinfarction, disabling stroke or ischemia-driven TVR at 6 months. There were no significant differences in the primary end points between the 2 primary angioplasty groups, neither were there differences between the 2 stenting groups. On the other hand, significant differences were observed in the combined primary end point at 6 months between primary PTCA and the stent group due to a lower rate of target vessel revascularization with stenting, with no differences in mortality or stroke. The apparent absence of relative benefit of abciximab with stenting at 6 months is due to the great relative weight of stenting in reducing restenosis, the most powerful component of the combined end point. The benefit of abciximab33 was demonstrated in the analysis of the results at 30 days.
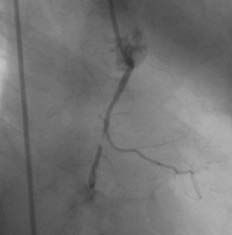
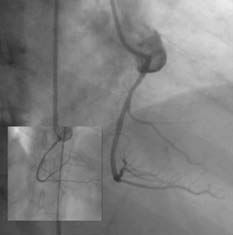
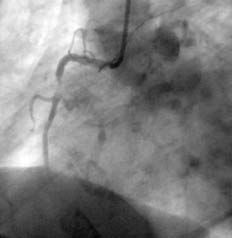
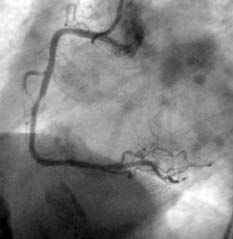
The ULTIMA registry34 analyzed the results of a subgroup of 40 patients with AMI due to left main coronary artery disease treated with stenting. It is currently accepted that stenting is used as a reference in percutaneous revascularization of the AMI culprit vessel. However, a reduction in TIMI grade 3 flow and an incidence of distal embolization occur in 15% of the patients. It has been thought that one way to decrease distal embolization could be direct stenting (Figures 1 and 2) without predilatation with balloon. Loubeyre et al35 presented a work which compared direct stenting with predilatation in 206 AMI patients.
Those in the first group had less incidence of slow-flow/no-reflow (P=.01) with better ST-segment resolution (P=.01), and no differences in mortality or hospital stay. Other authors have also confirmed good outcomes with direct stenting.36,37 The absence of predilatation reduces plaque fragmentation and distal embolization, thus preserving microcirculation with the consequent reduction in no-reflow events and the best myocardial reperfusion. It is currently believed that primary PTCA with direct stenting is advisable in lesions with a great amount of thrombotic material visualized via angiography, in young patients with suspicion of soft lesions, and in cases where thrombus aspiration devices were not used. When there are calcified lesions or when inadequate stent expansion is suspected, predilatation is recommended.
Figure 1. A) The right coronary artery with subocclusive stenosis in the middle third. B) Final result after direct stenting.
Outcome of Mechanical Reperfusion in Specific Clinical Contexts
The outcome is worse in AMI patients with diabetes than in non-diabetic patients. In a subanalysis of the CADILLAC study, Stuckey et al38 demonstrated that the use of abciximab does not improve outcome in these patients. However, there were significant differences in the group treated with stenting compared to patients treated with balloon angioplasty. Harjai et al39 showed that diabetic patients had a greater proportion of multiple-vessel disease, but had better TIMI grade 3 flow at admission. Hospital mortality was 4.6% versus 2.6% in non-diabetic patients (P=.005). During follow-up, there was a significant difference in mortality versus non-diabetic patients (P<.0001). High mortality in diabetic patients is due to the fact they have larger infarctions, with greater LVEF dysfunction and greater incidence of kidney failure.
There is much debate regarding the scope of percutaneous revascularization in patients with multiple vessel lesions in the AMI context. Pellizzon et al40 compared 2 groups of patients: those with revascularization in the AMI culprit artery only or together with other vessels. There were no differences in reinfarction and revascularization rates after 1 year. However, there was greater mortality in the group undergoing revascularization of other vessels (P=.029), with an insignificant trend toward an increase in cardiac mortality. The AHA/ACC41 guidelines advise against PTCA in other arteries in the case of multiple vessel lesions in the AMI context. However, with current improvements in materials, in patients in whom adequate collateral flow should be ensured or who have lesions in other vessels but where an increase in procedural difficulty is not expected, some authors revascularize other vessels during the same procedure and this decreases morbidity, hospital stay, and costs.
Around 3%-5% of aortocoronary vein grafts fail per year from the time of intervention, and 3% of the patients with previous surgery develop infarction.42 Stone et al43 analyzed 58 patients from the PAMI 2 study with AMI and previous myocardial revascularization surgery. In 55% of cases, the AMI culprit artery was an aortocoronary vein graft. In comparison with native artery revascularization, the grafts had TIMI grade 3 flow with more thrombus. The patients with previous surgery had greater hospital mortality than nonoperated patients, especially if the treated vessel was a graft.44 The anatomy of the graft and the large amount of thrombotic material are 2 factors that affect procedural outcome in these patients.
Are Drug-Eluting Stents Indicated?
With the advent of drug-eluting stents (DES) the incidence of restenosis has been reduced.45-47 Their use in AMI is still under study since it is feared that they might increase the incidence of acute/subacute stent thrombosis. Saia et al48 presented a consecutive series of patients where their safety was demonstrated with a 0% incidence of angiographic restenosis. Lemos et al49 presented a study on the short- and long-term benefits of DES in 369 patients, comparing them with conventional stents.
The patients in the second group received a greater proportion of glycoprotein IIb/IIIa inhibitors. There were no differences between groups in vessel patency, infarct size (enzyme markers), and short-term events, nor were there were differences in stent thrombosis. Regarding evolution, at 10 months the patients who received DES had less incidence of combined adverse events (P=.02), basically due to a reduced risk of reintervention (P=.01). In the multivariate analysis, DES was the only independent predictor of a reduction in combined adverse events (P=.03). These results should still be confirmed by randomized studies.
Epicardial Flow and Myocardial Reperfusion
Traditionally, the good outcome of primary angioplasty has been associated with obtaining normal epicardial flow (early and sustained TIMI grade 3) in the AMI culprit artery.50,51 Subsequently, it was found that the patency of the epicardial vessel was not enough and that, in addition to obtaining a TIMI grade 3 flow, adequate myocardial perfusion was also required.
Studies were published evaluating myocardial perfusion52 via contrast echocardiography,53 positron emission tomography,54 and magnetic resonance imaging55 where it was demonstrated that, despite obtaining TIMI grade 3 flow, adequate tissue reperfusion was not always achieved. Dibra et al56 analyzed TIMI myocardial perfusion (TMP) comparing patients with TMP 2/3 versus 0/1. Patients in the first group had smaller infarct size (P=.001), with a trend toward lower mortality per year. The patients treated with stenting had a greater proportion of TMP 2/3 than those treated with thrombolysis (P=.001).
Van't Hof et al57 introduced the term myocardial blush (MB) grade, an angiographic method to describe the effectiveness of myocardial perfusion that was validated in relation to the extent of ST-segment elevation resolution in the electrocardiogram (ECG). They found an inverse relationship between MB and time of ischemia, infarct size, and LVEF. Myocardial blush grade was associated with increased mortality as the degree of perfusion decreased.
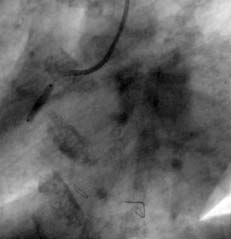
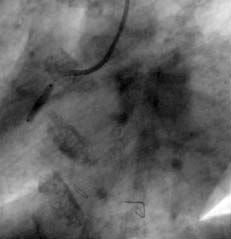
Stone et al58 evaluated the importance of myocardial perfusion. In 173 patients with AMI, TIMI grade 3 flow was obtained in 94.2%. However, of these, MB was normal in 29.4% of the patients. No patient with Another component of great value when evaluating reperfusion is ST-segment elevation resolution in the ECG after catheterization. The persistence of ST-segment elevation after recanalization reflects the presence of sustained transmural lesion and is correlated with alterations in reperfusion and microcirculation dysfunction involving great myocardial damage.57 Claeys et al59 studied the prognostic value of postcatheterization ST-segment resolution in 91 AMI patients. In 36% of patients, there was persistent ST-segment elevation after percutaneous revascularization. These patients developed more extensive infarction and had worse prognosis: gre ater cardiac death rate (P=.01) and other adverse events (P<.005). It could be considered that older patients would have greater endothelium dysfunction and, thus, greater ischemia/reperfusion injury in the microcirculation. Ischemia/reperfusion injury releases vasodilating and arterial hypotensive factors.60 The development of reperfusion injury is related to an increase in microvascular injury and arrhythmias and even sudden death and perpetuation of the thrombotic state. The microvasculature would be highly exposed to procoagulant factors, inhibition of the fibrinolytic system, and platelet aggregation, partly due to the reduction in nitric oxide, all of which would induce microvascular occlusion.61,62 This flow dysfunction leads to the no-reflow or slow-flow events first described in humans by Ito et al.63 Matetzky et al64 evaluated the persistence of ST-segment elevation in 117 patients. The ST segment was not resolved in 24% of patients and their LVEF was worse at discharge (P<.01). They also had greater mortality and heart failure in the long term (P=.004). The patients with no-reflow have a greater risk of infarction and death. The causative mechanisms are: arterial vasoconstriction, loss of capillary self-regulation, distal embolization, microvascular disorder, tissue edema, increased inflammatory mediators, endothelial alteration or increases in vasoconstrictor receptors such as angiotensin-II. No-reflow affects 10%-20% of the patients treated for AMI.65 Regarding its treatment, various drugs have been used such as verapamil, adenosine and, recently, sodium nitroprusside with the best results. THROMBUS MANAGEMENT Thrombectomy and Distal Protection Devices A common angiographic finding is thrombus associated with a lesion leading to difficulties in obtaining adequate myocardial perfusion. Most myocardial infarctions begin with the formation of thrombus on broken plaque and later occlusion of the artery. Despite significant progress in the development of antiplatelet aggregation and antithrombotic agents, the persistence of thrombus debris in lesions is strongly associated with a high risk of distal embolization and no-reflow events leading to poor angiographic and clinical outcomes. The mechanical approaches developed include thrombus aspiration catheters, rheolytic systems, ultrasound lysis, distal protection devices, and mechanical thrombectomy.66 X-Sizer Device The X-Sizer helical thrombectomy device (EV3, Minneapolis, Minnesota) (Figures 2C and D) has been effective in the treatment of acute coronary syndrome (ACS) with and without ST-segment elevation, coronary stent thrombosis, and saphenous vein grafts. Moreno et al67 report their initial experience of 4 patients with AMI who underwent removal of intracoronary thrombus. In the most extensive experiment involving several Spanish and Italian centers, the same level of safety and efficacy were obtained in a broad group of patients with AMI. Brueck et al68 reported a patient with aortocoronary bypass graft stenosis who underwent successful rescue thrombectomy without complications after the implantation of 2 stents for occlusion of the native artery; this fact suggests that the X-Sizer system is able to recover the thrombotic debris after macroembolization of the saphenous vein graft, including the native coronary artery. Kwok et al69 evaluated the first angiographic results in humans, involving 14 patients who underwent intracoronary thrombectomy with the X-Sizer system. The mean stenosis diameter was reduced by 89.3% to a final residual stenosis of 14.4%. There were no episodes of perforation, distal coronary spasm, abrupt closure or slow-flow/no-reflow. The X-TRACT70 study compared patients with ACS where the culprit lesion was in native coronary arteries or saphenous vein grafts. No differences were found regarding the primary end point (periprocedural infarction), but the patients in whom the X-Sizer system was used had a lower incidence of large periprocedural infarctions, especially when the target lesion was in native arteries.
Figure 2. A) Complete occlusion of the anterior descending coronary artery in the middle third. The passage of the X-Sizer through the occlusion can be seen in the box. B) Result after thrombectomy. The final outcome can be seen in the box after implanting the stent. C and D) X-Sizer system catheter and device.
Beran et al71 prospectively compared conventional PCI with pretreatment with the X-Sizer system in 65 patients with similar clinical characteristics, of whom 49 had AMI. It was found that in ACS with suspected thrombus, pretreatment with the X-Sizer system improved epicardial flow and accelerated ST-segment resolution compared to conventional PCI.
Napodano et al72 evaluated the effects of mechanical thrombectomy with the X-Sizer system on myocardial reperfusion during primary angioplasty in 92 patients with angiographic evidence of intraluminal thrombus, who were randomized for intracoronary thrombectomy followed by stenting or a conventional stenting strategy. Myocardial reperfusion was evaluated by myocardial blush and ST resolution. Thrombolysis In Myocardial Infarction grade 3 flow was similar in both groups. Myocardial blush grade 3 was found in 71.7% of the thrombectomy group patients and in 36.9% conventional strategy group (P=.006). ST-segment resolution >50% occurred more frequently in patients undergoing thrombectomy.
The XAMINE study compared the results of 200 patients in 14 European centers randomized to receive treatment with the X-Sizer system or conventional therapy in the AMI culprit artery, with clear evidence of thrombus and TIMI grade 0-1 flow in the initial coronary angiography. The primary end point was the magnitude of postprocedural ST-segment resolution which was significantly better in the group treated with the X-Sizer system. Thrombectomy was also more effective in reducing the incidence of distal embolization and slow-flow/no-reflow.73
AngioJet System
The AngioJet system (POSSIS Medical, Inc., Minneapolis, Minnesota) (Figure 3) has been used in a series of patients with AMI. In a study with 115 patients with AMI treated with the AngioJet system a 92% success rate was obtained; however, distal embolization occurred in 12%, sustained no-reflow in 5%, and coronary perforation in 3%.74
Figure 3. Console and catheters for rheolytic thrombectomy (AngioJet).
Nakagawa et al75 studied the efficacy and safety of the AngioJet catheter in 31 patients with AMI with angiographic follow-up at 3 and 6 months. The procedure was carried out successfully in 29 patients (94%). There were no major in-hospital events and none during follow-up.
The results of a randomized multicenter study done in the USA were recently published which compared rheolytic thrombectomy using the AngioJet system with conventional coronary intervention in patients with ST-segment elevation AMI. The AngioJet system was unable to decrease the infarct size compared to conventional intervention or improve ST-segment resolution. The use of rheolytic thrombectomy using the AngioJet system increased the incidence of complications, including mortality compared to conventional interventional therapy. In summary, the AngioJet system was less safe and effective than conventional intervention.76
Distal Protection Devices
PercuSurge GuideWire Device
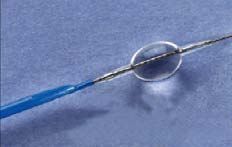
In several multicenter trials, the use of the PercuSurge GuideWire device has been effective in reducing the incidence of distal embolization in the treatment of degenerated saphenous vein grafts77,78 (Figure 4).
Figure. 4. PercuSurge. Distal protection used in the EMERALD study.
Belli et al79 evaluated the uthe PercuSurge device in combination with glycoprotein IIb/IIIa inhibitors in 8 patients with AMI and obtained a procedural success of 88%, without periprocedural complications. Wu et al80 evaluated angiographic results at 6 months and perfusion at the site of the distal protection balloon in patients with AMI. Seventy-four patients were treated with the PercuSurge device. It was found that the use of the PercuSurge device during PCI in the AMI context immediately led to a high rate of TIMI grade 3 flow in the epicardial vessels and preserved the integrity of the microcirculation.
In the EMERALD study, Stone et al81 included 501 patients with AMI who underwent primary or rescue angioplasty with and without the PercuSurge distal protection device. In this randomized multicenter study, the PercuSurge device was no more effective than conventional intervention for ST-segment resolution and microvascular flow; neither was it safer nor able to reduce mortality and total events.
FilterWire Ex Device
The FilterWire Ex device (FW) is another distal protection device (Figures 5 and 6). Some small observational studies have shown its efficacy in saphenous vein graft angioplasty.77,82,83 Stone et al84 conducted a randomized study in 651 patients with 682 saphenous vein graft lesions treated with angioplasty and FW or GuardWire. Procedural success was similar regarding epicardial flow, angiographic complications, and immediate major events or those at 30 days.
Limbruno et al85 evaluated the safety and efficacy of FW as an adjunct to primary angioplasty in 53 patients with AMI compared to a control group treated with conventional primary angioplasty.
The FW was associated with a higher occurrence of MB grade 3 and early ST-segment resolution. A large randomized study is needed to compare the results of primary angioplasty with and without distal protection with FW to determine the real part played by this device.
Figure. 5. A) Plaque with great thrombotic content in the align="center" CLASS=20Textogeneral>
Figure 6. FilterWire Ex Distal protection filter.
SPECIAL TECHNIQUES
Circulatory Hypothermia
Hypothetical reductions in infarct size achieved via circulatory hypothermia have been verified by Dixon et al86 in the COOL-MI study which compared patients undergoing conventional primary angioplasty with others who underwent systemic hypothermia in addition to primary angioplasty. Adverse events were similar in the 2 groups. No differences were found regarding the study's primary end point (infarct size), although in the anterior infarction subgroup, the infarct size was smaller in those patients where it was possible to achieve a deep level of hypothermia. One problem is that patients find this difficult to tolerate.
The future role of this technique is still to be determined, but it will be necessary to find a way to make it more comfortable and to find patient subgroups in which hypothermia can lead to significant clinical differences: large infarctions, late presentation, previous ventricular dysfunction, etc.
Hyperbaric Oxygen
Another technique87 employed in patients to reduce infarct size is the administration of hyperoxygenated blood (PO2=600) in the infarction culprit artery immediately proximal to the obstruction, following angioplasty, continuously over 90 min. The promising results of a small observational study were not confirmed by a randomized study where hyperbaric oxygen was not able to reduce the infarct size nor the incidence of adverse events, although the hyperbaric oxygen reduced the infarct size in patients with <6 h evolution of symptoms p PREVENTION OF DAMAGE DUE TO REPERFUSION
A matter that continues to be of concern regarding mechanical reperfusion is the prevention of reperfusion damage. Different theories have been proposed to explain this and, in turn, it has been thought that different therapeutic strategies could be used in its prevention. Among the therapies proposed are the administration of fluosol, magnesium, trimetazidine, cylexin, adenosine, anti-CD18, eniporide, etc.
Pexelizumab is one of the latest drugs proposed as being efficacious in preventing damage due to reperfusion which, via inhibiting complement C5, should reduce myocardial damage. Granger et al88 tested the safety and efficacy of pexelizumab in the COMMA study, where its effect was tested in 814 randomized patients. Although there were no significant differences in the primary end point, the infarct size, there was a difference in 6-month mortality in the patients treated with pexelizumab. This effect will be tested in a wider group of patients (>3500) in the APEX-AMI study that has already begun the inclusion period.
PRIMARY ANGIOPLASTY CENTERS
In many countries, due to decentralization and streamlining the health systems, there is a lack of centers with experience in primary PTCA and even hospitals without a catheterization laboratory or on-site cardiac surgery which could solve complications arising from percutaneous revascularization. For this reason, some authors and guidelines advocate the use of thrombolytic therapy in the patients who arrive at these centers, with referral to percutaneous revascularization or surgery as required. Based on this, some authors have suggested the creation of centers with experience in catheterization (lacking on-site cardiac surgery) or transfer to centers with greater expertise.
In the C-PORT89 study, PTCA was evaluated in centers without on-site cardiac surgery and revascularization was compared with lytic therapy. In the first case, the results were significantly better. The NRMI90 study compared revascularization in centers with and without on-site cardiac surgery. Mortality was independent of delay from onset of symptoms to hospital admission within a range of <2 to 12 h mortality however was related door-balloon time with a significant increase in when this exceeded 2 thus some authors consider that delays due transfer another center would 91
In the PAMI No SOS Study,92 Wharton et al compared patients admitted with AMI who underwent PTCA in centers without on-site cardiac surgery with those transferred to centers with these facilities. The door-balloon time was 201 min. There were no deaths during transferal. More beta-blockers, abciximab, and stents were used in the nontransferred group. The latter group obtained a final higher TIMI grade 3 flow (P=.004).
There were no significant differences in length of hospital stay. Nor were there significant differences in the primary end point at 30 days. However, mortality was greater in the transferred group (P=.043), although after adjustment for differences in baseline variables, these differences were not significant. There was no differences per year in the rates of reinfarction, disabling stroke or combined end points. Primary PTCA can be carried out safely in centers without on-site cardiac surgery with similar results. The AHA/ACC93 guidelines recommend revascularization in centers without on-site cardiac surgery, but with interventional cardiologists with proven experience (>75 cases/year).
Vessel patency and long-term evolution are not necessarily related to the time of PTCA.18,94 Grines et al95 analyzed the results of the Air PAMI Study, which compared on-site thrombolysis with transfers to another center for PTCA. The study finished prematurely with 138 patients. There were no deaths or need for resuscitation during transfer. The length of hospital stay was significantly shorter in the patients treated with PTCA. At 30 days there were no significant differences in the primary end point. However, in the multivariate analysis, transfer for PTCA was an independent predictor of decrease in the primary end point (P=.028).
The PRAGUE 196 study was designed to compare three strategies in patients with AMI <6 h from onset: a) thrombolysis in the hospital; b) thrombolysis and transfer for facilitated PTCA; and c) transportation to a center for PTCA without previous thrombolytic treatment. Three hundred patients were included in the study. There were no complications in the third group during transfer. The primary end point of death, reinfarction, and stroke at 30 days was less frequent in the third group (8%) compared to the second (15%) and first (23%; P<.02). The incidence of reinfarction was significantly reduced in the third group compared to the other two ( P<.03). Mortality was reducedfrom 14% in the first group to 7% in the third although the difference was not significant due to sample size. The results were similar in the first 2 groups.
In the PRAGUE 2 study, Widimsky et al97 evaluated 850 patients with AMI and compared their transfer to a center with a catheterization laboratory to on-site thrombolysis in the admission hospital. Patients were included with onset of symptoms <12 h and were <120 km away from the catheterization laboratory study ended prematurely due to excess mortality in group treat ed with thrombolysis after 3 h 2 5-fold i ncrease compared angioplasty complications during transfer ptca only occurred 1 hospital stay was shorter P<.05). Thirty-day mortality was 6.8% for the angioplasty group and 10% for thrombolysis group ( P=.12). The combined end point was less frequent in the PTCA group ( P<.003), with more stroke in the thrombolysis group ( P=.03).
The DANAMI 2 study98 compared on-site thromboly ti c treatment with transfer to another center for PTCA. It was stopped prematurely due to the demonstrated benefits of PTCA. Mean time from symptom onset to randomization was 135 min. There were no deaths during transferal. There was a 75% reduction in the relative risk of reinfarction (P=.0003) and decreased MACE at 30 days (P=.02) in the PTCA group.
The difference was significant regardless of the location of the infarction, whether inferior or anterior. Angioplasty was of benefit in the different groups depending on time from symptom onset to PTCA and was maintained despite the time of treatment (even >4 h). These studies demonstrate that the transfer of patients with AMI to centers that have catheterization laboratories yields better results, fewer adverse events, and better long-term prognosis than on-site thrombolysis.
Zijsltra et al11 carried out a metaanalysis of the randomized studies that compared transfer to another center for PTCA versus on-site thrombolysis. Mortality was 6.8% in the group transferred for PTCA versus 9.6% in the on-site thrombolysis group (P=.01). Keeley et al12 carried out a similar metaanalysis. Despite the delay in transfer, primary PTCA significantly reduced non-fatal reinfarction, stroke, and other adverse events.
The term facilitated angioplasty refers to the use of pharmacological agents to achieve reperfusion before arrival at the catheterization laboratory. The aim is to achieve a greater number of patients with the AMI culprit artery open upon arrival at the admitting hospital and to preserve ventricular function while avoiding an increase in complications. Although in absolute terms facilitated angioplasty would include any pharmacological treatment, the term is associated with glycoprotein IIb/IIIa inhibitor therapy with half-doses of the thrombolytic drug.
Stone et al99 analyzed the importance of TIMI grade 3 flow upon arrival at the catheterization laboratory based on patients included in the PAMI studies. Some 2507 patients were included and 16% had TIMI grade 3 flow in the initial coronary angiography. These patients were compared to those with smaller TIMI flow. In the former, LVEF was greater (57 vs 53%; P=.003) and were less prone to develop heart failure (P=.009). There were differences in mortality at 6 months (P=.009). These results point to the advisability of facilitated PTCA.
The HEAP study100 did not find the use of high-dose heparin of benefit as pretreatment for angioplasty. The PACT study101 compared fibrinolytic treatment versus placebo (plus PTCA in both groups) in 606 patients with AMI. Sixty-one percent of patients in the first group had the artery open upon arrival at the catheterization laboratory (33% with TIMI grade 3 flow) versus 34% in the second group (P=.001). However, there were no differences in obtaining TIMI grade 3 flow at the end of the trial. O'Neill et al102 compared primary versus facilitated PTCA (previous infusion of streptokinase).
Thrombolysis In Myocardial Infarction grade 2-3 was similar in both groups (92% vs 98%). During follow-up there was a trend toward better LVEF in the second group. However, in the latter patients a large increase in hemorrhage requiring transfusion was found (39%). Similarly, the ECSG study103 found no increase in benefit with facilitated PTCA, whereas there was an increase in risk of adverse events.
Similar results were obtained in the SWIFT study.104 In the CAPTIM study, Bonnefoy et al105 compared treatment with prehospital fibrinolysis (n=419) to primary PTCA (n=421). There were no significant differences between groups in the combined end point (8.2% vs 6.2%) of death, non-fatal reinfarction, and non-fatal disabling stroke, nor were there differences in mortality.
The GRACIA 1 study106 compared 2 postinfarction strategies following thrombolysis: a) invasive: systematic coronary angiography and revascularization (if indicated); and b) coronary angiography following an ischemia-guided conservative approach.
The study demonstrated the superiority of systematic angiography after fibrinolysis, but it does not shed more light on the relationship between primary and facilitated angioplasty. The GRACIA-2 study107 compared primary PTCA with stenting with facilitated PTCA in 212 patients with AMI <12 h the size of sample was not sufficient to obtain differences in clinical results p In this study, no differences were found between the 2 strategies regarding infarct size (measured by biological markers) or LVEF at 6 weeks, neither were there significant differences in wall-motion index at 6 weeks. While the results of larger randomized studies are awaited, it can currently be stated that facilitated PTCA does not provide benefits compared to transfer and revascularization without previous fibrinolysis. The AHA/ACC guidelines 41 establish as a class IIb recommendation for facilitated PTCA in high-risk patients where PTCA will be delayed and there is a low risk of complications due to bleeding.
SCIENTIFIC SOCIETY THERAPEUTIC GUIDELINES
The guidelines of the European Society of Cardiology108 establish as a class I recommendation (level of evidence A) the treatment of AMI with PTCA if instituted <90 min from contact with the physician or admission center and carried out in centers proven experience p The AHA/ACC 41 guidelines consider as a class I recommendation PTCA within 90 min from diagnosis of infarction in AMI <12 h and carried out in centers with proven expertise if symptom duration is <3 h ptca is recommended in the event that procedure carried out <1 h from diagnosis if the patient arrives 3 h from symptom onset PTCA is recommended as the treatment of choice. Percutaneous transluminal coronary angioplasty is also recommended in patients with heart failure or pulmonary edema and symptom onset <12 h when the onset of ami symptoms is 12 h PTCA is recommended in the case of heart failure, electric or hemodynamic instability, or persistent ischemic symptoms.
The Clinical Practice guidelines of the Spanish Society of Cardiology109 establish as a class I indication primary angioplasty for patients with AMI <12 h from symptom onset until admission to a hospital with angioplasty facilities and proven experience in especially extensive ami hemodynamic instability or contraindications for thrombolytic treatment p Patients <75 years old in cardiogenic shock and within the first 6 h of symptom onset are also included class iia indications those with extensive ami contraindication for thrombolytic treatment admitted to a hospital without angioplasty facilities whose transfer permits p Patients with extensive AMI or hemodynamic instability admitted to a center with similar characteristics and whose transfer and intervention is not delayed by >120 min. Indications for class III include patients with non-extensive AMI admitted to hospitals without angioplasty facilities.
RESCUE PTCA/CONTRAINDICATION FOR THROMBOLYSIS
Rescue PTCA is the mechanical reopening of the vessel after failed thrombolysis. Ross et al110 did not find significant differences between rescue and primary PTCA regarding mortality or restenosis. The RESCUE study111 evaluated 151 patients with anterior AMI treated with thrombolysis with angiographic control at 6-8 h from pain onset with TIMI grade 0-1 flow.
Cases of cardiogenic shock were excluded. The patients were divided into 2 groups depending on whether they had undergone angioplasty (n=78) or conservative treatment (n=73). Left ventricular function improved significantly in those receiving angioplasty (P=.04). There were differences in severe heart failure although none in total mortality (P=.05).
The benefit of mechanical reperfusion with angioplasty after failed thrombolysis would be independent of myocardial preservation and would be related to better ventricular remodeling, a lower incidence of heart failure, greater electric stability, and the probable later development of collateral circulation.
Hong et al112 presented a study with 31 patients who had undergone salvage PTCA and compared them to primary PTCA patients. There was a stronger trend toward arterial hypotension (P=.021) in the first group, implying that more patients were admitted to the catheterization laboratory in cardiogenic shock. There were no differences in survival at 1, 6, and 12 months.
The European Society of Cardiology108 guidelines establish as a class IIa recommendation (level of evidence B) rescue PTCA when thrombolysis fails or when this is contraindicated.
PERCUTANEOUS TRANSLUMINAL CORONARY ANGIOPLASTY IN POSTINFARCTION ANGINA
Postinfarction angina (PIA) is defined as angina appearing between 24 h and 30 days following acute AMI. This is a clear marker of poor prognosis and is associated with greater mortality.113,114 Thrombolytic treatment has not reduced the incidence of PIA.115
In the GUSTO-I study, 20% of the patients presented PIA. Risk of reinfarction before 30 days was higher in those who presented electrocardiographic changes concomitant with angina, although mortality increased only in those with hemodynamic alterations.116
The GISSI-3 APPI study117 demonstrated that myocardial revascularization in these patients reduces the incidence of later events. In the GRACIA registry118 a significant reduction in mortality was found in revascularized patients.
The AHA/ACC guidelines recommend invasive therapy only if there is evidence of ischemia after effective thrombolysis. In stable patients this is a class III indication.41
CARDIOGENIC SHOCK
The estimated incidence of cardiogenic shock in patients with AMI is approximately 7%-10%.119,120 It is an important cause of death within the AMI context.121-123 Cardiogenic shock is directly related to infarction size.124,125 A 40% loss of the myocardium of the left ventricle irremediably originates cardiogenic shock.126,127 Symptoms can be due to extensive AMI, reinfarction in the same area, or a small infarction in a patient with previous ventricular dysfunction.
The mechanical complications of the infarction, such as ruptured papillary muscle, ventricular septal defects or left ventricle free wall rupture, are also causes of cardiogenic shock.126,127 Catheterization laboratory support is urgently required.125 However, the only beneficial treatment is early myocardial revascularization. In the GUSTO-1 study,120 successful PTCA in cases of shock was associated with a reduction in 30-day mortality. In the ULTIMA registry, 15% of the patients were admitted with AMI or cardiogenic shock. Hospital mortality in these cases was 69%.26
In the SHOCK study,122 2 treatment strategies were compared in 320 patients with cardiogenic shock: revascularization versus medical treatment. Although there was no significant reduction in 30-day mortality in the revascularization group, there was at 6 months. In the patients <75 years old there was an absolute reduction in mortality of 15 at 30 days and 20 6 months however the patients 75 years old, there was a 22% increase in mortality in those who underwent revascularization versus those who received medical treatment.
In the SHOCK registry128, hospital mortality decreased from 71% in 1992 to 60% in 1997. In the revascularized patients in the registry, hospital mortality was 50% in 1992 and 38.5% in 1997. In a study by Moreno et al,129 in the patients admitted in cardiogenic shock, survival improved from 36.4% before 1994 to 76.5% in 1997. Sanborn et al130 demonstrated that LVEF, initial TIMI flow grade, the number of diseased vessels, and the culprit vessel were significantly related to 1-year survival in patients with cardiogenic shock.
Zeymer et al131 presented the results of the ALKK registry which evaluated predictors of mortality in 1333 patients with AMI complicated by cardiogenic shock who were treated in 80 centers in Germany. In the multivariate analysis, left anterior descending coronary artery disease, 3-vessel disease, TIMI flow <3 after catheterization age and delay in establishing treatment were independent predictors of mortality p The GRACIA registry 132 evaluated the results of stenting in patients with cardiogenic shock. Total hospital mortality was 59%. This was less in patients 2 underwent revascularization than in those 2 underwent conservative therapy (45% vs 69%; P<.001). In the multivariate analysis, presenting with cardiogenic shock and revascularization with stenting were the two predictors of hospital survival. In two small registries the benefits of stenting compared to balloon PTCA were found in patients in cardiogenic shock. 133,134
The European Society of Cardiology108 guidelines establish as a class I recommendation (level of evidence C) revascularization and the use of intraaortic balloon counterpulsation (IABP) for AMI complicated by cardiogenic shock. The AHA/ACC41 guidelines establish as a class I recommendation PTCA in patients <75 years with ami who develop cardiogenic shock within 36 h from admission and can benefit revascularization the first 18 of symptom onset percutaneous transluminal coronary angioplasty is a class iia recommendation when patients are 75 years old.
Intraaortic balloon pump counterpulsation is theoretically beneficial in high-risk patients, since it increases cardiac output, reduces oxygen consumption, and increases coronary flow.135 However, in very compromised patients, the increase in heart output is modest (Vranckx P, Serruys PW. Personal communication. PCR, 2004). Current indications in patients with AMI extend to those who present ventricular septal rupture and mitral valve insufficiency,136 postinfarction angina, refractory ventricular arrhythmias,137 and progressive left ventricular failure.
138 In AMI patients it can maintain and improve hemodynamic parameters while the left ventricle recovers,139 it reduces the incidence of recurrent ischemia,140 and the incidence of reocclusion of the AMI culprit artery.141 Stone et al142 created a registry with 5495 consecutive AMI patients between 1996 and 2001. Major complications related to the use of IABP (critical limb ischemia, severe bleeding, or death) occurred in 2.7% of patients. Death was directly attributed to IABP in 3 patients (0.05%).
The AHA/ACC guidelines41 include as a class I indication IABP in AMI as a stabilizing measure in cases of cardiogenic shock, acute mitral valve insufficiency or ventricular septal rupture after AMI and as a stabilizing measure for myocardial revascularization; recurrent and intractable ventricular arrhythmias, accompanied by hemodynamic instability; and in case of postinfarction angina refractory to conventional treatment.
Cardiac Support Devices
Temporary cardiac support devices make it possible for the damaged heart to recover while maintaining optimal tissue perfusion and decreasing ventricular load. Thus, one of the factors that negatively affects patient evolution is eliminated. Mechanical unloading of the myocardium during ischemia and reperfusion decreases workload and myocardial oxygen consumption, thus increasing benefit, since the infarct size is related to the degree of reduction in left ventricle load. Although there is little experience in this regard, good observational results have been found in patients in cardiogenic shock with very serious ventricular failure (Vranckx P, Serruys PW. Personal communication. PCR, 2004).
ACKNOWLEDGEMENTS
We would like to thank Dr Luis García-Nielsen and Dr José Pinto for their excellent help in the preparation of this manuscript.
Section Sponsored by the Dr. Esteve Laboratory
Correspondence: Dr. E. García.
Hemodinámica y Cardiología Intervencionista. Hospital Universitario Gregorio Marañón.
Dr. Esquerdo, 46. 28007 Madrid. España.
E-mail: ejgarcia@retemail.es