Proprotein convertase subtilisin/kexin 9 (PCSK-9) inhibitors are a new lipid-lowering therapy that has been conclusively demonstrated to reduce both low-density lipoprotein cholesterol (LDLc) and major cardiovascular events.1,2 Evolocumab and alirocumab have been available for more than 1 year, and consequently there is still scarce evidence of their effectiveness in real-world practice under local or national reimbursement indications. The aim of our study was to describe the safety and effectiveness (in terms of LDLc reduction) of this treatment in the initial patients treated with either evolocumab or alirocumab in 5 institutions.
We performed a retrospective study of patients treated with evolocumab or alirocumab in 5 different hospitals in Spain in the first 6 months that these treatments were locally available. There are 3 current indications for reimbursement in Spain: familial hypercholesterolemia (FH) without cardiovascular disease (CVD) but LDLc> 100 mg/dL, CVD but LDLc> 100mg/dL despite maximum tolerated lipid-lowering therapy and statin intolerance and LDLc> 100 mg/dL. Statin treatment was classified according to current guidelines.3 Patients with intolerance were defined as those who abandoned statins due to adverse effects or did not tolerate high-doses.
Absolute and relative differences between biochemical determinations were calculated and differences were assessed by 1-way ANOVA and postestimation Bonferroni test. All analyses were performed using STATA 14.3 (Stata Corp 2009. College Station, TX: StataCorp LP).
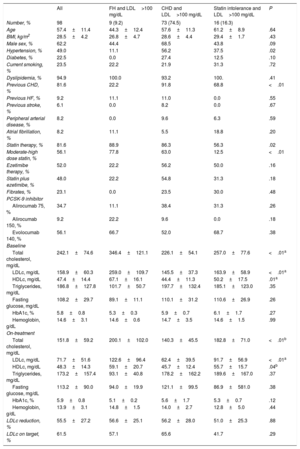
We identified 98 patients referred for PCSK-9 treatment initiation but 1 patient refused to start it and 14 patients did not undergo a second blood test; therefore, safety and efficacy could be analyzed in 83 patients. The most frequent indication was CVD and LDLc> 100 mg/dL (74.5%); statin intolerance (16.3%) and FH and LDLc> 100mg/dL (9.2%) were less frequent. No relevant differences were observed between the 3 patient subgroups other than a slightly higher prevalence of male sex and hypertension in patients with CHD (Table 1). Baseline statin use was very high and more than half of the patients were receiving ezetimibe. The most frequently used PCSK-9 inhibitor was evolocumab.
Clinical features of the population according to indication for PSCK-9 therapy
| All | FH and LDL>100 mg/dL | CHD and LDL>100 mg/dL | Statin intolerance and LDL>100 mg/dL | P | |
|---|---|---|---|---|---|
| Number, % | 98 | 9 (9.2) | 73 (74.5) | 16 (16.3) | |
| Age | 57.4±11.4 | 44.3±12.4 | 57.6±11.3 | 61.2±8.9 | .64 |
| BMI, kg/m2 | 28.5±4.2 | 26.8±4.7 | 28.6±4.4 | 29.4±1.7 | .43 |
| Male sex, % | 62.2 | 44.4 | 68.5 | 43.8 | .09 |
| Hypertension, % | 49.0 | 11.1 | 56.2 | 37.5 | .02 |
| Diabetes, % | 22.5 | 0.0 | 27.4 | 12.5 | .10 |
| Current smoking, % | 23.5 | 22.2 | 21.9 | 31.3 | .72 |
| Dyslipidemia, % | 94.9 | 100.0 | 93.2 | 100. | .41 |
| Previous CHD, % | 81.6 | 22.2 | 91.8 | 68.8 | <.01 |
| Previous HF, % | 9.2 | 11.1 | 11.0 | 0.0 | .55 |
| Previous stroke, % | 6.1 | 0.0 | 8.2 | 0.0 | .67 |
| Peripheral arterial disease, % | 8.2 | 0.0 | 9.6 | 6.3 | .59 |
| Atrial fibrillation, % | 8.2 | 11.1 | 5.5 | 18.8 | .20 |
| Statin therapy, % | 81.6 | 88.9 | 86.3 | 56.3 | .02 |
| Moderate-high dose statin, % | 56.1 | 77.8 | 63.0 | 12.5 | <.01 |
| Ezetimibe therapy, % | 52.0 | 22.2 | 56.2 | 50.0 | .16 |
| Statin plus ezetimibe, % | 48.0 | 22.2 | 54.8 | 31.3 | .18 |
| Fibrates, % | 23.1 | 0.0 | 23.5 | 30.0 | .48 |
| PCSK-9 inhibitor | |||||
| Alirocumab 75, % | 34.7 | 11.1 | 38.4 | 31.3 | .26 |
| Alirocumab 150, % | 9.2 | 22.2 | 9.6 | 0.0 | .18 |
| Evolocumab 140, % | 56.1 | 66.7 | 52.0 | 68.7 | .38 |
| Baseline | |||||
| Total cholesterol, mg/dL | 242.1±74.6 | 346.4±121.1 | 226.1±54.1 | 257.0±77.6 | <.01a |
| LDLc, mg/dL | 158.9±60.3 | 259.0±109.7 | 145.5±37.3 | 163.9±58.9 | <.01a |
| HDLc, mg/dL | 47.4±14.4 | 67.1±16.1 | 44.4±11.3 | 50.2±17.5 | .01a |
| Triglycerides, mg/dL | 186.8±127.8 | 101.7±50.7 | 197.7±132.4 | 185.1±123.0 | .35 |
| Fasting glucose, mg/dL | 108.2±29.7 | 89.1±11.1 | 110.1±31.2 | 110.6±26.9 | .26 |
| HbA1c, % | 5.8±0.8 | 5.3±0.3 | 5.9±0.7 | 6.1±1.7 | .27 |
| Hemoglobin, g/dL | 14.6±3.1 | 14.6±0.6 | 14.7±3.5 | 14.6±1.5 | .99 |
| On-treatment | |||||
| Total cholesterol, mg/dL | 151.8±59.2 | 200.1±102.0 | 140.3±45.5 | 182.8±71.0 | <.01b |
| LDLc, mg/dL | 71.7±51.6 | 122.6±96.4 | 62.4±39.5 | 91.7±56.9 | <.01a |
| HDLc, mg/dL | 48.3±14.3 | 59.1±20.7 | 45.7±12.4 | 55.7±15.7 | .04b |
| Triglycerides, mg/dL | 173.2±157.4 | 93.1±40.8 | 178.2±162.2 | 189.6±167.0 | .37 |
| Fasting glucose, mg/dL | 113.2±90.0 | 94.0±19.9 | 121.1±99.5 | 86.9±581.0 | .38 |
| HbA1c, % | 5.9±0.8 | 5.1±0.2 | 5.6±1.7 | 5.3±0.7 | .12 |
| Hemoglobin, g/dL | 13.9±3.1 | 14.8±1.5 | 14.0±2.7 | 12.8±5.0 | .44 |
| LDLc reduction, % | 55.5±27.2 | 56.6±25.1 | 56.2±28.0 | 51.0±25.3 | .88 |
| LDLc on target, % | 61.5 | 57.1 | 65.6 | 41.7 | .29 |
BMI, body mass index; CHD, coronary heart disease; FH, familial hypercholesterolemia; HDLc, high-density lipoprotein cholesterol; HF, heart failure; LDL, LDLc, low-density lipoprotein cholesterol; PCSK-9, proprotein convertase subtilisin/kexin 9.
Unless otherwise indicated, the data are expressed as No. (%) or mean±standard deviation.
LDLc ontarget was considered <100mg/ dL in patients with FH and <70mg in the other 2 columns.
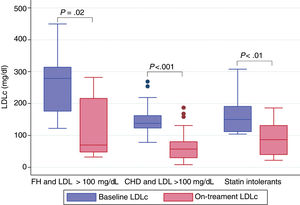
LDLc at the time when a PCSK-9 inhibitor was indicated (baseline LDLc) was 158.9 (60.3) mg/dL. The on-treatment second blood test was obtained at a median interval of 184.0 days (interquartile range, 93.5-310.5). The mean LDLc reduction was 55.5%. As shown in Figure 1, the treatment was effective all 3 groups and no differences were observed between them (P=.88). Nonetheless, patients treated with evolocumab had higher reductions than those treated with alirocumab: 67.7% vs 40.7% (P <.001), despite having the same baseline LDLc; only 9 patients received alirocumab 150mg, baseline LDLc was 177.0mg/dL, and LDLc reduction was equipotent to the 75mg dose. No relevant adverse effects were reported; only 1 patient complained of a local site reaction after the seventh dose of evolocumab, which disappeared after we switched the site of administration.
Our initial experience with PCSK-9 inhibitors in real-world patients is in agreement with previously reported efficacy and safety.1,2 We believe this reported experience is representative and relevant for daily clinical practice. PCSK-9 inhibitors are a completely novel and complementary strategy for LDLc reduction that can be used as monotherapy or combined with any other lipid-lowering drugs.4 The positive results of randomized clinical trials1,2 will most likely change guideline recommendations for drug combinations and upgrade recommendations for their use.
Evolocumab and alirocumab seem to have a similar effect on LDLc reduction at equipotent doses.1,2 Alirocumab has 2 different presentations and most patients in the ODYSSEY Outcomes trial received the 75mg dose2; therefore, final on-treatment LDLc was 53.3mg/dL2 in contrast to 30 mg/dL in the FOURIER trial.1 Because most patients in our study treated with alirocumab received the 75mg dose, the effect on LDLc reduction was lower than that for patients treated with evolocumab. The cost-effectiveness of PCSK-9 inhibitors in real-world practice is a matter of debate,5 but we believe that our results could help the implementation of these therapies in daily practice.
As with every early experience with novel drugs, our study has the limitation of a small sample size and a retrospective and observational design. Moreover, treatment adherence to PCSK-9 inhibitors and the other lipid-lowering drugs was self-reported. Baseline LDLc was fairly high, which might reflect the fact that treatment was recommended in selected patients with very high LDLc.
In conclusion, the initial experience with evolocumab and alirocumab in real-world patients with uncontrolled LDLc supports the efficacy and safety of PCSK-9 inhibitors. Mean LDLc reduction was 55.5% and was statistically higher in patients treated with evolocumab vs alirocumab, as expected because of the doses chosen by the clinicians. Our study reinforces the efficacy of PCSK-9 inhibitors in daily clinical practice.
CONFLICTS OF INTERESTThe authors report no conflict of interest related to this manuscript. The investigators received the support of the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV).