Cardiac arrest that occurs during long-distance running races is most commonly attributed to hypertrophic cardiomyopathy or atherosclerotic coronary disease. Conduction system disease is an extremely rare cause of sudden death in athletes and the final cause remains unknown in a small proportion of cases.1 We present a case of malignant bradyarrhythmia in a long distance runner.
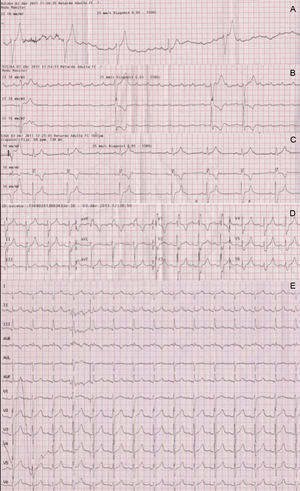
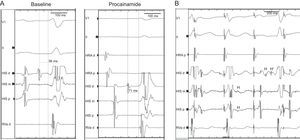
A 46-year-old healthy man, with no previous cardiovascular history, had a cardiac arrest immediately after completing a half marathon. At the finish line, he fainted and was attended by the emergency medical services, which initiated advanced life support in the presence of severe bradycardia (23bpm) and hypotension. A first electrocardiogram showed complete atrioventricular block (AVB) with slow wide QRS complex escape (Figure 1A). Intravenous atropine only increased sinus rate without improving the degree of AVB (Figure 1B) and therefore a transcutaneous pacemaker was placed and the patient was transferred to our hospital. Forty-five minutes after cardiac arrest, atrioventricular (AV) conduction progressively recovered (Figure 1C), until 1:1 AV conduction resumed with first degree AVB and complete right bundle branch block (Figure 1D). At hospital admission, the electrocardiogram only showed a borderline prolongation of the PR interval (210ms) (Figure 1E). Diagnostic work-up including blood analyses, echocardiogram, and coronary angiography ruled out drug use, electrolyte disturbances, myocardial damage (maximum troponin T, 0.03ng/mL), structural heart disease, and coronary lesions. An electrophysiologic study was performed documenting the presence of a bordeline HV interval (56ms) and a notched wide His deflection (35ms) (Figure 2A). After procainamide infusion (10mg/kg), there was an HV interval prolongation up to 70ms with further His widening (45ms). Atrial pacing at progressively higher rates demonstrated the presence of intermittent conduction between the proximal (H) and the distal His bundle potentials (H’) (Figure 2B).
A: First electrocardiogram recorded following consciousness recovery showed complete atrioventricular block and wide QRS escape. B: Intravenous atropine increased sinus rate but did not improve atrioventricular conduction, strongly suggesting an infranodal location of the atrioventricular block. C: Partial atrioventricular conduction recovery 45min after the cardiac arrest. D: Right bundle branch block was observed during the first few minutes following 1:1 atrioventricular conduction recovery. E: Electrocardiogram at hospital admission.
A: Electrophysiologic study performed 3 days after the index admission: slightly prolonged HV interval (56ms) and notched His at baseline, which further prolonged following procainamide challenge. B: Atrial pacing 380ms cycle length following procainamide infusion shows a proximal His bundle deflection (H) with intermittent failure to conduct to the distal His bundle (H’) (second paced beat) and split His potentials (third paced beat).
With the diagnosis of second degree intra-His block unmasked by procainamide infusion,2 a double chamber pacemaker was implanted. During the year following the episode, the patient continued running everyday free of symptoms. An exercise test performed 6 months after the episode showed a normal baseline electrocardiogram and the development of frequency-dependent right bundle branch block at a critical rate that resolved with sinus rate slowing. Genetic testing excluded mutations in the principal ion channel genes associated with progressive cardiac conduction disease (SNC5A, KCNQ1, KCNH2, KCNE1, and KCNJ2).
Hypertrophic cardiomyopathy and ischemic heart disease are the most common causes of cardiac arrest during long distance competitions. However, in a non-negligible proportion of cases (5%-10%), the ultimate cardiovascular cause of death remains unknown.1 Exercise-induced complete AVB is an uncommon event and, to our knowledge, has not been previously reported as a cause of cardiac arrest during exercise.1 Our case highlights the clinical importance of this phenomenon as a possible cause of sudden death in athletes. The prolonged duration of the AVB episode (>1h) and the unstable and wide QRS complex escape suggest that, if the patient had not been immediately resuscitated, fatal asystole might have developed at any time during the episode.
Although exercise-induced ischemia in patients with coronary artery disease or coronary vasospasm can cause AVB, in other instances it may occur in the absence of ischemia in patients with an otherwise normal resting electrocardiogram.3 In the latter group, infranodal location is the most common site of block.2,3 The present case recapitulates the electrophysiological features of an intra-His conduction impairment unmasked by procainamide infusion and atrial pacing: prolonged intra-His duration, notched His deflection, and split potentials with intermittent conduction.2 The development of rate-dependent right bundle branch block several months after the admission points to a progressive conduction disease etiology. However, the reasons for the development of these conduction disturbances in a previously healthy, middle-aged, well-trained patient remain unknown. Gene mutations have been implicated with the development of progressive cardiac conduction disease, and the number is ever-growing.4 Cardiac sarcoidosis and giant cell myocarditis are responsible for some unexplained AVB in young adults,5 but these possibilities seem unlikely in the absence of symptoms or myocardial damage. Recent magnetic resonance imaging studies have suggested a link between lifelong endurance exercise and myocardial fibrosis that could eventually have consequences on the conduction pathways6 and could constitute an explanation for our findings.