Conventional risk scores have not been accurate in predicting peri- and postprocedural risk of patients undergoing transcatheter aortic valve implantation (TAVI). Elevated levels of the tumor marker carbohydrate antigen 125 (CA125) have been linked to adverse outcomes after TAVI. We studied the additional value of CA125 to that of the EuroSCORE in predicting long-term mortality after TAVI.
Methods and resultsDuring a median follow-up of 59 weeks, 115 of 422 patients (27%) died after TAVI. Mortality was higher with elevated CA125 (> 30 U/mL) and EuroSCORE (> median) (47% vs 20%, P<.001 and 38% vs 16%, P<.001, respectively). In the multivariable analysis, CA125 (> 30 U/mL) remained an independent predictor of mortality (hazard ratio [HR], 2.16; 95% confidence interval [95%CI], 1.48-3.15; P<.001) and improved the predictive capability of the model (C-statistic: 0.736 vs 0.731) and the net reclassification index (51% 95%CI, 33-73) with an integrated discriminative improvement of 3.5% (95%CI, 0.5-8.4). A new variable (CA125-EuroSCORE) was created, with the combinations of the 2 possible binary states of these variables (+, elevated, -, not elevated; C1: CA125- EuroSCORE-; C2: CA125+ EuroSCORE-; C3: CA125- EuroSCORE+; C4: CA125+ EuroSCORE+). Patients in C1 exhibited the lowest cumulative mortality rate (14% [26 of 181]). Mortality was intermediate for C2 (CA125 > 30 U/mL and EuroSCORE ≤ median) and C3 (CA125 ≤ 30 U/mL and EuroSCORE > median): 27% (8 of 30) and 28% (37 of 131), respectively. Patients in C4 (CA125 > 30 U/mL and EuroSCORE > median) exhibited the highest mortality (55% [44 of 80], P-value for trend<.001).
ConclusionsCA125 offers additional prognostic information beyond that obtained by the EuroSCORE. Elevation of both markers was associated with a poor prognosis.
Keywords
Risk prediction in transcatheter aortic valve implantation (TAVI) remains very challenging in elderly patients with a high comorbidity burden. Identifying patients at high, or even prohibitive risk, in whom the procedure may be futile, is likely to have important implications for patient selection.1
Several scores have been used for risk prediction in TAVI.2 The European System for Cardiac Operative Risk Evaluation (EuroSCORE),3 which was developed for standard cardiac surgery, is frequently used in the setting of TAVI. However, this score has limited value, because TAVI-specific variables and predictors are lacking. As a result, the EuroSCORE provides reliable predictive capacities for extreme values only.4 Therefore, the search for objective tools to improve risk stratification in TAVI, such as biomarkers, remains an unmet need.5
Tumor marker carbohydrate antigen 125 (CA125) is an emerging cardiac biomarker. In heart failure, CA125 has been shown to be associated with the degree of fluid overload, inflammation, clinical severity, and adverse outcomes, independently of established risk factors including natriuretic peptides and cardiorenal function.6–8 In TAVI, our group has recently shown that elevated CA125 values, at baseline as well as longitudinally assessed, are independently associated with adverse outcomes.9 Whether this biomarker adds a clinically relevant improvement to the prognostic information obtained by conventional risk scores is unknown.
Therefore, we explored the added value of baseline CA125 measurements in combination with the EuroSCORE for the prediction of long-term mortality after TAVI.
METHODSPatient Population and Description of Transcatheter Aortic Valve Implantation ProceduresThis prospective observational study included 512 consecutive patients who underwent TAVI for severe aortic stenosis at the University of Regensburg Medical Center from July 2009 to March 2014. CA125 was determined using a chemiluminometric immunoassay (ADVIA Centaur, Siemens, Germany) at least 24hours before TAVI. CA125 was not obtained in 90 patients due to logistic reasons. Therefore, the final study population consisted of 422 patients. The logistic EuroSCORE for each patient was calculated as published.3
The interdisciplinary Heart Team discussed all patients and decided on the therapeutic strategy in each patient. The study protocol was approved by the local ethics committee and all patients provided written informed consent to participate in a clinical registry.
TAVI was performed via the transfemoral (n=237) or the transapical approach (n=180). In 5 patients, a different access route was used (subclavian [n=1] or direct aortic [n=4]). Access route was dichotomized to ‘transfemoral’ vs ‘nontransfemoral’. Procedures were performed in the catheterization laboratory or the hybrid-operating suite under general anesthesia.
Follow-up and Definition of OutcomesThe endpoint of this study was time to all-cause death after TAVI. Patients who were alive on last contact were censored. Device success, procedural success, and procedural complications were categorized using criteria defined by the Valve Academic Research Consortium.10
All data were prospectively collected. Two cardiologists and 3 trained nurses, unaware of CA125 values, performed follow-up on a 3-monthly basis either by scheduled visits in the outpatient clinic, telephone interviews with patients or their families, or by reviewing the patients hospital records.
Statistical AnalysisContinuous variables are expressed as the mean (± standard deviation) or as the median (interquartile range) and were compared using the unpaired Student t test (or Mann-Whitney U test as appropriate). Discrete variables were compared with the chi-square test or Fisher's exact test as appropriate.
For dichotomic analysis, CA125 was divided according to the manufacturer's cut-off value for a pathologic test result (30 U/mL), which has previously been shown to possess prognostic information after TAVI.9 For the EuroSCORE, the population was dichotomized according to the median value (≥ 13.9%). A new variable (CA125-EuroSCORE) was created, with the combinations of the 2 possible binary states of these variables (+, elevated, -, not elevated; C1: CA125- EuroSCORE-; C2: CA125+ EuroSCORE-; C3: CA125- EuroSCORE+; C4: CA125+ EuroSCORE+).
To assess for potential selection bias, the mortality rate of patients with (n=422) and without (n=90) available CA125 measurements were compared using the Kaplan-Meier method.
Time to death according to elevated CA125 and EuroSCORE as well as CA125-EuroSCORE was estimated using the Kaplan-Meier method with differences tested by the log-rank test. To assess the independent association of CA125 and EuroSCORE and of CA125-EuroSCORE categories with mortality, a Cox proportional hazard regression analysis was performed with computation of hazard ratios (HR) with their 95% confidence intervals (95%CI). The first model included only CA125 and EuroSCORE. The second model was adjusted for EuroSCORE and, since the EuroSCORE already summarizes important predictors of mortality, further adjustment was performed using those variables with a P<.05 in the univariate analysis and not already included in the EuroSCORE. These variables were diabetes, atrial fibrillation, previous pacemaker implantation, and elevated NYHA class (III or IV).
The incremental prognostic usefulness of CA125 to the multivariable model was evaluated by calculating the integrated discrimination improvement (IDI) and the net reclassification improvement with the corresponding 95%CIs. These statistical indices have been developed to evaluate the added predictive capability of a new marker and have been described in detail elsewhere.11 In brief, the IDI can be viewed as the difference between improvement in average sensitivity and any potential increase in average ‘one minus specificity’. The net reclassification improvement sums up differences in the proportion of individuals moving up minus the proportion moving down for people who develop events, and the proportion of individuals moving down minus the proportion moving up for people not developing events.11 The discriminative accuracy and model calibration were assessed by the Harrell's C, Somer's D, and the Hosmer-Lemeshow tests, respectively.
A 2-sided P-value of < .05 was considered statistically significant for all analyses. The SPSS statistical package (Version 22.0, SPSS Inc., Chicago, Illinois, United States) and Stata (StataCorp. 2011. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) were used.
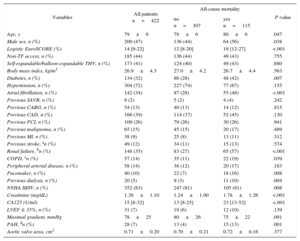
RESULTSBaseline characteristics of the entire patient population and according to all-cause mortality are displayed in Table 1. Overall, 422 patients underwent TAVI and had available baseline CA125 measurements.
Baseline Characteristics for the Entire Population and by All-cause Mortality
| Variables | All patients n=422 | All-cause mortality | P-value | |
|---|---|---|---|---|
| no n=307 | yes n=115 | |||
| Age, y | 79±6 | 79±6 | 80±6 | .047 |
| Male sex, n (%) | 200 (47) | 136 (44) | 64 (56) | .038 |
| Logistic EuroSCORE (%) | 14 [9-22] | 12 [8-20] | 19 [12-27] | <.001 |
| Non-TF access, n (%) | 185 (44) | 136 (44) | 49 (43) | .755 |
| Self-expandable/balloon-expandable THV, n (%) | 173 (41) | 124 (40) | 49 (43) | .680 |
| Body mass index, kg/m2 | 26.9±4.3 | 27.0±4.2 | 26.7±4.4 | .563 |
| Diabetes, n (%) | 134 (32) | 86 (28) | 48 (42) | .007 |
| Hypertension, n (%) | 304 (72) | 227 (74) | 77 (67) | .155 |
| Atrial fibrillation, n (%) | 142 (34) | 87 (28) | 55 (48) | <.001 |
| Previous SAVR, n (%) | 9 (2) | 5 (2) | 4 (4) | .242 |
| Previous CABG, n (%) | 54 (13) | 40 (13) | 14 (12) | .815 |
| Previous CAD, n (%) | 166 (39) | 114 (37) | 52 (45) | .130 |
| Previous PCI, n (%) | 109 (26) | 79 (26) | 30 (26) | .941 |
| Previous malignoma, n (%) | 65 (15) | 45 (15) | 20 (17) | .489 |
| Previous MI, n (%) | 38 (9) | 25 (8) | 13 (11) | .312 |
| Previous stroke, an (%) | 49 (12) | 34 (11) | 15 (13) | .574 |
| Renal failure, bn (%) | 148 (35) | 83 (27) | 65 (57) | <.001 |
| COPD, cn (%) | 57 (14) | 35 (11) | 22 (19) | .039 |
| Peripheral arterial disease, n (%) | 58 (14) | 38 (12) | 20 (17) | .183 |
| Pacemaker, n (%) | 40 (10) | 22 (7) | 18 (16) | .008 |
| Previous dialysis, n (%) | 20 (5) | 9 (3) | 11 (10) | .004 |
| NYHA III/IV, n (%) | 352 (83) | 247 (81) | 105 (91) | .008 |
| Creatinine (mg/dL) | 1.38±1.10 | 1.24±1.00 | 1.78±1.26 | <.001 |
| CA125 (U/ml) | 15 [8-32] | 13 [8-25] | 23 [13-52] | <.001 |
| LVEF ≤ 35%, n (%) | 31 (7) | 19 (6) | 12 (10) | .139 |
| Maximal gradient, mmHg | 78±25 | 80±26 | 75±22 | .091 |
| PAH, dn (%) | 28 (7) | 13 (4) | 15 (13) | .001 |
| Aortic valve area, cm2 | 0.71±0.20 | 0.70±0.21 | 0.72±0.16 | .377 |
CA125, tumor marker carbohydrate antigen 125; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; TF, transfemoral; THV, transcatheter heart valve.
Unless otherwise indicated, values are presented as mean±SD or as median [interquartile range].
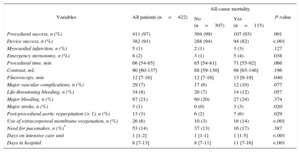
During a median follow-up of 59 (range, 34-107) weeks, 115 patients died (27%). The patients who died during follow-up were older, were more frequently male, showed a higher prevalence of diabetes, atrial fibrillation, chronic obstructive pulmonary disease, NYHA class III or IV, and more often had a previous pacemaker and worse renal function. Patients who died during follow-up experienced more procedural and in-hospital complications (Table 2). There was no difference in mortality between patients treated via the transfemoral and nontransfemoral approach (Table 1). Exclusion of the 5 patients with an alternative access route did not substantially affect the results.
Intra- and Postprocedural Data and Complications of the Entire Patient Population and by All-cause Mortality
| Variables | All patients (n=422) | All-cause mortality | P-value | |
|---|---|---|---|---|
| No (n=307) | Yes (n=115) | |||
| Procedural success, n (%) | 411 (97) | 304 (99) | 107 (93) | .001 |
| Device success, n (%) | 382 (91) | 288 (94) | 94 (82) | <.001 |
| Myocardial infarction, n (%) | 5 (1) | 2 (1) | 3 (3) | .127 |
| Emergency sternotomy, n (%) | 8 (2) | 3 (1) | 5 (4) | .038 |
| Procedural time, min | 66 [54-85] | 65 [54-81] | 71 [55-92] | .066 |
| Contrast, mL | 90 [60-137] | 88 [59-130] | 98 [65-146] | .196 |
| Fluoroscopy, min | 12 [7-16] | 12 [7-16] | 13 [9-19] | .040 |
| Major vascular complications, n (%) | 29 (7) | 17 (6) | 12 (10) | .077 |
| Life-threatening bleeding, n (%) | 34 (8) | 20 (7) | 14 (12) | .057 |
| Major bleeding, n (%) | 87 (21) | 60 (20) | 27 (24) | .374 |
| Major stroke, n (%) | 3 (1) | 0 (0) | 3 (3) | .020 |
| Post-procedural aortic regurgitation (> 1), n (%) | 13 (3) | 6 (2) | 7 (6) | .029 |
| Use of extracorporeal membrane oxygenation, n (%) | 26 (6) | 10 (3) | 16 (14) | <.001 |
| Need for pacemaker, n (%)* | 53 (14) | 37 (13) | 16 (17) | .387 |
| Days on intensive care unit | 1 [1-2] | 1 [1-1] | 1 [1-5] | <.001 |
| Days in hospital | 8 [7-13] | 8 [7-11] | 11 [7-16] | <.001 |
Unless otherwise indicated, values are presented as mean±standard deviation or as the median [interquartile range].
Baseline characteristics and Kaplan-Meier estimates of mortality of patients with unavailable CA125 due to logistic reasons (n=90) did not differ from those in patients included in the study, indicating no relevant selection bias (Table 1 and Figure of the supplementary material).
Patients who died during follow-up had a significantly higher median logistic EuroSCORE than survivors (19% [12-27] vs 12% [8-20]; P<.001) and had significantly higher CA125 values (23.4 U/mL [13.1-52] vs 13.2 U/mL [7.5-24.5]; P<.001).
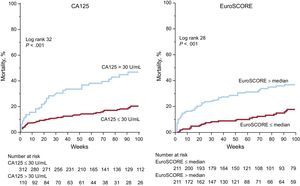
Elevated CA125 (> 30 U/mL) was present in 26% (110 of 422) of the patients and was associated with a significantly higher rate (47% [52 of 110], vs 20% [63 of 312]; P<.001) and risk of mortality (HR2.76; 95%CI, 1.91-3.99; P<.001). Mortality differences with CA125 > 30 U/mL were observed during the entire follow-up period, and were already tangible within the first months after TAVI (Figure 1, left panel).
Cumulative mortality according to CA125 and EuroSCORE. The cumulative mortality during follow-up according to elevated CA125 (left panel) and to EuroSCORE (right panel) is displayed. CA125, tumor marker carbohydrate antigen 125; EuroSCORE, European System for Cardiac Operative Risk Evaluation.
In parallel, patients with an elevated EuroSCORE (> median) exhibited a significantly higher rate (38% [81 of 211] vs 16% [34 of 211]; P<.001, Figure 1, right panel) and risk of mortality (HR2.83; 95%CI, 1.89-4.23; P<.001).
In the bivariate analysis with both the EuroSCORE as a continuous and as a categorized variable, elevated CA125 (> 30 U/mL) remained an independent predictor of mortality (HR2.26; 95%CI, 1.54-3.30; P<.001 and HR2.18; 95%CI, 1.53-3.20; P<.001, respectively). The C-statistic for predicting mortality with CA125 alone was lower than that of the EuroSCORE (0.657 vs 0.684).
Multivariable Analysis and Incremental Prognostic Value of CA125In the multivariable analysis, adjusted for EuroSCORE, diabetes, atrial fibrillation, previous pacemaker and elevated NYHA class, CA125 (> 30U/mL) persisted as an independent predictor of mortality (HR2.16; 95%CI, 1.48-3.15; P<.001).
No interaction was found between elevated CA125 and EuroSCORE, neither as a continuous (P for interaction .952) nor as a dichotomic variable (P, .917) indicating a homogenous effect of CA125 across EuroSCORE strata.
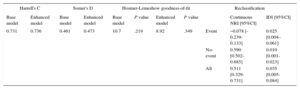
Addition of CA125 (> 30 U/mL) to the multivariable model marginally improved the predictive capability (C-Statistic: 0.736 vs 0.731). However, the addition of CA125 led to a significant improvement in the net reclassification index (51% 95%CI, 33-73) and to an integrated discriminative improvement of 3.5% (95%CI, 0.5-8.4, Table 3 and Table 2 of the supplementary material).
Improvement in Risk Stratification by CA125
| Harrell's C | Somer's D | Hosmer-Lemeshow goodness-of-fit | Reclassification | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Base model | Enhanced model | Base model | Enhanced model | Base model | P value | Enhanced model | P value | Continuous NRI [95%CI] | IDI [95%CI] | |
| 0.731 | 0.736 | 0.461 | 0.473 | 10.7 | .219 | 8.92 | .349 | Event | −0.078 [-0.239-0.133] | 0.025 [0.004–0.061] |
| No-event | 0.590 [0.502-0.685] | 0.010 [0.001-0.023] | ||||||||
| All | 0.511 [0.329-0.731] | 0.035 [0.005-0.084] | ||||||||
95%CI, 95% confidence interval; CA125, tumor marker carbohydrate antigen 125; IDI, integrated discrimination improvement, NRI, net reclassification improvement.
The base model includes EuroSCORE, atrial fibrillation, previous pacemaker, diabetes mellitus, and NYHA III or IV. Enhanced model CA125 > 30 U/mL was added.
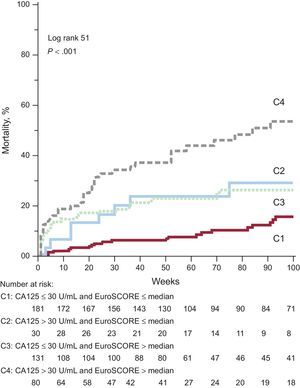
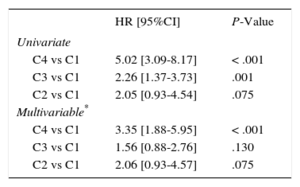
By combining the information of CA125 (dichotomized at 30 U/mL) and the EuroSCORE (dichotomized at the median, Figure 2), those patients in C1 (CA125 ≤ 30 U/mL and EuroSCORE ≤ median) exhibited the lowest cumulative mortality rate (14%, 26 of 181). Mortality was intermediate for C2 (CA125 > 30 U/mL and EuroSCORE ≤ median) and C3 (CA125 ≤ 30 U/mL and EuroSCORE > median): 27% (8 of 30) and 28% (37 of 131), respectively. Patients in C4 (CA125 > 30 U/mL and EuroSCORE > median) exhibited the highest mortality (55% [44 of 80], P-value for trend<.001). In the univariate and multivariable Cox regression analysis, the highest risk was found in patients with both risk markers elevated (C4) and intermediate risk was found when only 1 variable was elevated (C2 and C3, see Table 4).
Cumulative mortality combining CA125 and EuroSCORE. The cumulative mortality during follow-up according to the combination of CA125 and EuroSCORE is displayed. For details see text. CA125, tumor marker carbohydrate antigen 125; EuroSCORE, European System for Cardiac Operative Risk Evaluation.
Univariate and Multivariable Cox Regression Analysis for Mortality Risk Combining CA125 and the EuroSCORE
| HR [95%CI] | P-Value | |
|---|---|---|
| Univariate | ||
| C4 vs C1 | 5.02 [3.09-8.17] | < .001 |
| C3 vs C1 | 2.26 [1.37-3.73] | .001 |
| C2 vs C1 | 2.05 [0.93-4.54] | .075 |
| Multivariable* | ||
| C4 vs C1 | 3.35 [1.88-5.95] | < .001 |
| C3 vs C1 | 1.56 [0.88-2.76] | .130 |
| C2 vs C1 | 2.06 [0.93-4.57] | .075 |
95%CI, 95% confidence interval; CA125, tumor marker carbohydrate antigen 125; HR, hazard ratio; NYHA,New York Heart Association.
The main findings of this study confirm and expand our previous experience with CA125 as a prognostic marker in TAVI in a considerably larger cohort than previously published. The present study extends the prognostic usefulness of this biomarker by showing that CA125 offers incremental prognostic information beyond that provided by a commonly used risk score. A combination of CA125 and the EuroSCORE significantly improved risk prediction as measured by the net reclassification improvement and the IDI. Patients with elevated EuroSCORE and CA125 values exhibited a poor prognosis while those with low values had a relatively good prognosis.
Risk Prediction in Transcatheter Aortic Valve Implantation–Current Risk Scores and ShortcomingsThe selection of patients suitable for TAVI, in whom the expected benefits of the intervention outweigh its risk, remains a challenge. Despite successful TAVI, patients are still at high risk for adverse events due to a very high comorbidity burden and the natural prognosis remains unchanged in some patients despite a successful procedure.12 This issue is evident from the relatively high mortality rates reported from follow-up of randomized trials13 and registries,14 which are comparable to that in the present study. By using parameters available at baseline, the identification of those patients who will most likely benefit from the procedure as well as the certain proportion of patients with a poor prognosis, in whom a more conservative treatment may be beneficial or cost-effective, has been highlighted before.1
Several attempts to predict outcome after TAVI have been undertaken and scores have been developed to enhance risk prediction. The EuroSCORE, initially developed to estimate in-hospital mortality after cardiac surgery,3 summarizes a wide range of patient-, cardiac-, and surgical-related factors and has also been validated for the prediction of perioperative complications and long-term outcomes after cardiac surgery.15,16 In TAVI, the EuroSCORE has been widely adopted and is recommended for preprocedural risk assessment.17 However, some important TAVI-specific variables and predictors are not included, and 30-day mortality in these high-risk and elderly patients is markedly overestimated.18,19 Moreover, the predictive capability of the EuroSCORE seems to be only accurate for extreme values.4 Recently, the EuroSCORE II20 has been introduced to improve surgical risk prediction. However, especially in the higher risk tertiles of the TAVI population, no relevant improvement was achieved over EuroSCORE I21,22 and several studies have shown that current risk scores do not provide acceptable predictive ability.23–25 In some settings, the EuroSCORE II has even been shown to be inferior in risk prediction to the EuroSCORE I.25,26 Several other attempts have been undertaken to create TAVI-specific risk scores on the basis of clinical parameters,27–29 but the search for improvement in risk prediction continues.30
The Role of Biomarkers for Risk Prediction in Transcatheter Aortic Valve ImplantationBiomarkers might constitute a helpful tool for inclusion in novel risk scores for TAVI to improve prediction of outcomes and to discriminate patients who will derive the greatest benefit. Natriuretic peptides have been tested for risk prediction in TAVI with conflicting results.31–33 Previously, we found an association of NT-pro BNP with all-cause mortality after TAVI, which disappeared after adjustment for CA125 and other covariates.9 The short half-life34 and the high prevalence of old age and renal dysfunction in the TAVI population may explain the limited use of natriuretic peptides for prognostic purposes. Elevation of cardiac troponins after TAVI has also been associated with negative outcomes.35 However, since this information is obtained after the procedure has been carried out, this marker is not suitable for baseline risk prediction.
CA125 is an emerging biomarker in heart failure and is associated with disease severity and progression.36–38 It is released by mesothelial cells in response to serosal effusions and to proinflammatory cytokines.39 In addition, high CA125 levels have been shown to be present in up to 65% of patients admitted for acute heart failure and are strongly associated with a higher mortality risk.6,7 In patients with symptomatic aortic stenosis, CA125 is elevated and correlates with symptom severity and outcome.40 This marker is widely available, cost-effective, possesses a long half-life and does not seem to be relevantly influenced by age, renal function, or body mass index.6,7
Previously, our group has shown that elevated CA125 values at baseline and longitudinally assessed after TAVI are associated with adverse outcomes.9 The present data confirm these prior findings in a considerably larger cohort and further clarify the role in refining risk prediction.
We found CA125 to be a strong and independent prognostic marker for long-term mortality even after multivariable adjustment. The information obtained by this marker at baseline significantly improved the predictive capacity of the EuroSCORE. Compared with the complex multivariable EuroSCORE, a single CA125 measurement at baseline had a comparable predictive capacity for mortality after TAVI as reflected by the C-statistic and results from the univariate analysis. This predictive capability remained unchanged even after rigorous multivariable adjustment by the EuroSCORE and other strong predictors of adverse outcome after TAVI. Further analyses revealed that the addition of this biomarker to the EuroSCORE improved risk stratification by reclassification of half of the population, mainly by reclassifying patients not experiencing an event. The additional prognostic value of CA125 appeared to be more relevant in patients with elevated risk in terms of an elevated EuroSCORE.
Clinical ImplicationsThe mechanisms linking elevated CA125 levels with an increased mortality risk after TAVI are unclear. Elevated CA125 levels have been observed in the presence of greater fluid overload and proinflammatory activity and are also strongly related to disease severity and adverse outcomes in heart failure.6–8 Therefore patients with elevated CA125 at baseline may be regarded as those patients with more severe heart failure. Patients with elevated EuroSCORE are those with a high comorbidity burden. When the information on elevated EuroSCORE (patients with high comorbidity) with elevated CA125 (patients with advanced stage of heart failure) is combined, the benefit from TAVI may be limited, as reflected by the substantial mortality rate in this study (about 55% mortality within 2 years after TAVI). On the other hand, the use of CA125 identifies comorbid patients without biochemical evidence of cardiac failure who display lower mortality than expected by the EuroSCORE alone.
LimitationsOur data show a lack of benefit of TAVI in terms of mortality in patients with both elevated CA125 and elevated EuroSCORE. However, we cannot exclude a benefit of TAVI in other important measures like quality of life and symptomatic improvement in this patient population. Therefore, any conclusions regarding the appropriateness of TAVI in these patients should be drawn with care.
Although CA125 appears useful for baseline risk stratification in TAVI, there is a need for further clarification of its functional implications and whether normalization of elevated CA125 values by intensified treatment before TAVI impacts prognosis.
CONCLUSIONSCA125 offers incremental prognostic information beyond that provided by the EuroSCORE and significantly improves risk prediction. Elevation of both markers confers poor prognosis.
- -
Conventional risk scores have not been accurate in predicting peri- and postprocedural risks in patients undergoing TAVI. Elevated CA125 levels have been linked to adverse outcomes after TAVI.
- -
The present study extents the prognostic usefulness of this biomarker by showing that CA125 offers incremental prognostic information beyond that provided by a commonly used risk score. Combining CA125 to the EuroSCORE significantly improved risk prediction as measured by the net reclassification improvement and the IDI. Patients with both elevated EuroSCORE and CA125 values exhibited a poor prognosis, while those with low values had an excellent prognosis.
T.F. Lüscher received research grants to the institution from AstraZeneca, Bayer Healthcare, Biosensors, Biotronik, Boston Scientific, Medtronic, MSD, Merck, Roche and Servier, including lecture fees.
This project was supported in part by grants of the Swiss National Science Foundation (SPUM 33CM30-124112); Abbott Inc. Europe the Foundation for Cardiovascular Research – Zurich Heart House, Zurich, and the grants PIE15/00013 (ISCIII and FEDER funds), RD12/0042/0010 (FEDER) and FIS 15/00837 from the Instituto de Salud Carlos III.