Left-sided infective endocarditis with blood culture-negative has been associated with delayed diagnosis, a greater number of in-hospital complications and need for surgery, and consequently worse prognosis. The aim of our study was to review the current situation of culture-negative infective endocarditis.
MethodsWe analyzed 749 consecutive cases of left-sided infective endocarditis, in 3 tertiary hospitals from June 1996 to 2011 and divided them into 2 groups: group I (n=106), blood culture-negative episodes, and group II (n=643) blood culture-positive episodes. We used Duke criteria for diagnosis until 2002, and its modified version by Li et al. thereafter.
ResultsAge, sex, and comorbidity were similar in both groups. No differences were found in the proportion of patients who received antibiotic treatment before blood culture extraction between the 2 groups. The interval from symptom onset to diagnosis was similar in the 2 groups. The clinical course of both groups during hospitalization was similar. There were no differences in the development of heart failure, renal failure, or septic shock. The need for surgery (57.5% vs 55.5%; P=.697) and mortality (25.5% vs 30.6%; P=.282) were similar in the 2 groups.
ConclusionsCurrently, previous antibiotic therapy is no longer more prevalent in patients with blood culture-negative endocarditis. This entity does not imply a delayed diagnosis and worse prognosis compared with blood culture-positive endocarditis. In-hospital clinical course, the need for surgery and mortality are similar to those in patients with blood culture-positive endocarditis.
Keywords
.
IntroductionBlood cultures are a fundamental tool in the diagnosis and treatment of infective endocarditis (IE). Blood culture-negative imply the loss of a microbiological reference which might guide the therapy and diagnosis of the disease. Blood culture-negative endocarditis (BCN-E) has been associated with a delay in diagnosis, a greater number of intrahospital complications, greater need for surgery and, in short, a worse prognosis.1, 2, 3, 4, 5, 6 According to published data, the main cause of BCN-E has classically been prior administration of antibiotics.5, 7, 8, 9, 10, 11, 12
In the last decade, a series of changes has occurred in the epidemiological factors and microorganisms causing IE (higher patient age, predominance of Staphylococcus aureus, increased incidence of nosocomial IE, etc.).8, 13, 14, 15, 16, 17 Furthermore, in recent years, the use of transesophageal echocardiogram (TEE) in patients with suspected IE is increasingly frequent and early. All these changes have modified some diagnostic and prognostic aspects of this disease. The aim of our study was to review the current situation of BCN-E.
MethodsWe analyzed all consecutively recorded cases of IE in 3 tertiary hospitals occurring between June 1996 and June 2011. The episodes were recorded on a datasheet and were added to a general database prospectively and consecutively, in an orderly fashion, following the guidelines defined by the group of participating researchers and evaluated in annual meetings. Of the 896 IE episodes included in this period, only patients with left endocarditis (n=749) were selected, which composed our study group. Episodes of right endocarditis were excluded due to their different epidemiological, microbacteriological, clinical and prognostic profiles.
Episodes of left endocarditis were classified into 2 groups: group I (n=106), episodes of BCN-E, and group II (n=643), blood culture-positive (BCP-E) episodes. The diagnosis of endocarditis was performed according to the classic criteria of Duke University18 until 2002. From that date onwards, we used the modified version by Li et al.19 Of group I episodes, 74.5% (n=79) met the criteria for definite IE and 25.5% (n=27) for possible IE. Within this latter group, most episodes (n=24) showed a major criterion, determined by echocardiographic findings and 2 minor criteria. Only 3 episodes were diagnosed as possible IE with 3 minor criteria.
The information obtained from each patient was prospectively entered into our database following a standardized protocol for the 3 participating centers, which included performing at least 3 blood cultures at the time of admission and 48h after the start of antibiotic treatment. In most episodes, the antibiotic treatment was initiated empirically after blood cultures had been taken, following the recommendations of clinical practice guidelines.20, 21 Later, the antibiotic treatment was adjusted according to the results of the blood cultures. Three pairs of blood cultures were taken at each extraction and were incubated for up to 4 weeks. In all patients, the material obtained during surgery (valve tissue, prosthetic material, abscesses, peripheral embolisms, etc.) was delivered to the microbiology department. In BCN-E episodes, as well as in many other cases, some of this material was sent to the pathology department for histological evaluation with special dyes (Gram, silver, etc.). In episodes in which the blood cultures remained negative 72h after admission, serology was systematically requested for Brucella, Legionella, Coxiella, Mycoplasma and Chlamydia and, in later years, Bartonella.
To study the evolution of IE within our cohort and determine the possible effect of the time factor on the microbacteriological profile and its influence on the disease's behavior, we performed a series of subanalyses, dividing the study period into 3 intervals of similar lengths (period 1: 1996-2001; period 2: 2002-2005; period 3: 2006-2011). We also studied the microbacteriological profile within each of the defined intervals.
Situations or procedures that held a risk of bacteremia and occurred 2 months before symptom onset were considered possible entry sites. Previous antibiotic treatment was considered relevant in episodes that occurred 15 days prior to blood culture extraction.
A transthoracic echocardiogram and a TEE were performed in all patients. The echocardiographic criteria used to define the presence of vegetations, abscesses, pseudoaneurysms and fistulas has been described in other publications.22, 23 The size of vegetations was determined by measuring their largest diameter, and in cases with more than 1 vegetation, the largest was used.
Indications for surgery during the active phase of the disease were established at the beginning of the study by means of a consensus among the researchers and included heart failure refractory to medical treatment, signs of persistent infection (defined as persistence of bacteremia or fever lasting more than 7 days after the start of appropriate antibiotic treatment, and exclusion of secondary or metastatic sources of the infection), fungal infection, and recurrent embolism despite appropriate antibiotic treatment. In patients who did not undergo surgery despite its being indicated, the reason was the patient's refusal or excessive surgical risk according to the EuroSCORE scale after 1999, and before that date, the decision made by a multidisciplinary team which included at least 1 clinical cardiologist and a cardiac surgeon.
A total of 62 epidemiological, clinical, electrocardiographic, radiological, echocardiographic, microbiological and prognostic variables were recorded.
Statistical AnalysisThe data are expressed as absolute frequencies or as percentages in the case of qualitative variables. The quantitative variables are described as mean (SD) and the median and the interquartile range in the case of asymmetry. To compare qualitative variables, either the chi-square or Fisher's exact test were used, as indicated. The quantitative variables were compared using Student t test and its non-parametric equivalent, the Mann-Whitney U test. Statistical significance was set at P<.05. The software package used for the analysis was SPSS version 15.0 for Windows (SPSS, Inc., Chicago, Illinois, United States).
Results Epidemiology and Clinical PresentationThe mean age of our population (n=749) was 61 (16) years. Some 63.2% were men and 38.5% were from other centers. In 40% of patients, the site of infection was a valve prosthesis (early prosthetic IE: 34%, and late prosthetic IE: 65.6%). The demographic variables are compared in Table 1. There were no statistically significant differences between the 2 groups in comorbidity or predisposing heart disease. The period from symptom onset to diagnosis of IE was similar in both groups. Disease presentation was acute (<15 days) in 48% of the patients and chronic (>3 months) in 16%. No significant differences were found in the ratio of patients that had received antibiotic treatment 15 days prior to admission between the 2 groups (Table 1).
Table 1. Demographic Variables, Predisposing Factors, Comorbidities, Entry Site, and Interval Between Symptom Onset and Diagnosis.
| Epidemiological characteristics | Negative BC(n=106) (Group I) | Positive BC(n=643) (Group II) | OR (95%CI) | P |
| Age, years | 61±16 | 62±14 | 1.01 (0.99-1.02) | .283 |
| Symptoms until admission, days | 34±41 | 42±83 | 1 (0.99-1.01) | .544 |
| Origin | 40.6% (43) | 37.8% (241) | 1.12 (0.74-1.71) | .584 |
| Gender, male | 65.1% (69) | 63.1% (406) | 1.09 (0.71-1.67) | .699 |
| Community-acquired | 70.2% (73) | 65.6% (417) | 0.86 (0.54-1.36) | .330 |
| Nosocomial | 24.8% (26) | 27.1% (173) | 0.26 (0.03-1.93) | .664 |
| Prior cardiopathy | 68.9% (73) | 66.1% (425) | 1.12 (0.72-1.74) | .658 |
| Period from symptoms onset to diagnosis | ||||
| >3 months | 17% (18) | 15% (95) | Reference | .808 |
| 2-3 months | 8.5% (9) | 6.6% (42) | 0.92 (0.39-2.21) | .858 |
| 1-2 months | 16% (17) | 14% (89) | 1.04 (0.51-2.12) | .922 |
| 15 days-1 month | 14.2% (15) | 15.6% (99) | 1.29 (0.62-2.69) | .492 |
| <15 days | 44.3% (47) | 48.7% (309) | 1.30 (0.73-2.32) | .374 |
| Comorbidities | ||||
| Diabetes | 15.1%(16) | 22.1% (142) | 0.63 (0.36-1.10) | .102 |
| Alcohol use | 2.9% (3) | 6.9% (44) | 0.40 (0.12-1.30) | .116 |
| Immunosuppressant treatment | 6.7% (7) | 6.3% (40) | 1.07 (0.47-2.46) | .871 |
| Chronic anemia | 13.3% (14) | 19.8% (127) | 0.62 (0.34-1.13) | .114 |
| Chronic renal failure | 11.4%(12) | 10.5% (67) | 1.10 (0.57- 2.12) | .767 |
| Dialysis | 4% (2) | 2.1% (6) | 1.99 (0.39-10.13) | .331 |
| Cancer | 6.7% (7) | 9.7% (62) | 0.67 (0.30-1.15) | .322 |
| COPD | 7.6% (8) | 8.3% (53) | 0.92 (0.42-1.98) | .820 |
| Collagenopathy | 1.9% (2) | 1.7% (11) | 1.10 (0.24-5.02) | >.999 |
| Immunosuppression | 5.7% (6) | 7.7% (49) | 0.73 (0.30-1.75) | .481 |
| Antibiotic prophylaxis | 16.2% (11) | 16.5% (67) | 0.98 (0.49-1.96) | .947 |
| Previous antibiotic treatment | 40% (36) | 35.2%(192) | 1.23 (0.78-1.94) | .375 |
| Entry site | ||||
| Local infection | 5.7% (6) | 12% (77) | 0.44 (0.19-1.04) | .054 |
| Previous surgery | 10.4% (11) | 12.8% (82) | 0.79 (0.4-1.54) | .485 |
| Dental manipulation | 11.3% (12) | 6.9% (44) | 1.73 (0.88-3.40) | .107 |
| Genito-urinary tract | 0% (0) | 3.7% (24) | 0.85 (0.83-0.88) | .037 |
| Digestive tract | 2.8% (3) | 3.1% (20) | 0.90 (0.26-3.09) | >.999 |
| Intravenous catheter | 2.8% (3) | 10.1% (65) | 0.26 (0.08-0.84) | .016 |
| Clinical presentation | ||||
| Fever | 67.9% (72) | 82.6% (530) | 0.45 (0.28-0.71) | <.001 |
| Cardiologic | 53.8% (57) | 40.1% (257) | 1.74 (1.15-2.63) | .008 |
| Neurologic | 16% (17) | 17.5% (112) | 0.90 (0.52-1.57) | .717 |
| Pulmonary | 6.7% (7) | 9% (58) | 0.72 (0.32-1.62) | .422 |
| Rheumatologic | 8.5% (9) | 12.8% (82) | 0.63 (0.31-1.3) | .210 |
| Renal | 5.7% (6) | 6.9% (44) | 0.81 (0.34-1.96) | .646 |
| Cutaneous | 3.8% (4) | 7.3% (47) | 0.50 (0.17-1.40) | .178 |
| Constitutional syndrome | 25.7% (27) | 32.4% (208) | 0.72 (0.45-1.15) | .168 |
| Abdominal | 8.5% (9) | 7.8% (50) | 1.09 (0.52-2.30) | .811 |
| Signs/symptoms upon admission | ||||
| New murmur | 44.3% (47) | 46.5% (298) | 0.92 (0.61-1.39) | .681 |
| Shivers | 36.3% (33) | 47.4% (270) | 0.63 (0.40-0.99) | .048 |
| Heart failure | 48.6% (51) | 37.8% (242) | 1.55 (1.03-2.35) | .036 |
| Fever upon admission | 57.5% (61) | 73.4% (470) | 0.49 (0.32-0.75) | .001 |
| Fever prior to admission | 59.2% (58) | 72.3% (431) | 0.55 (0.36-0.86) | .008 |
| Dyspnoea | 50.5% (53) | 43.2% (277) | 1.34 (0.89-2.02) | .165 |
| Renal failure | 16% (17) | 15.3% (98) | 1.06 (0.60-1.86) | .838 |
| Septic shock | 0.9% (1) | 6.4% (41) | 0.14 (0.02-1.03) | .024 |
| Thoracic pain | 15.1% (16) | 14.2% (91) | 1.08 (0.60-1.91) | .802 |
| Abdominal pain | 9.4% (10) | 10.9% (70) | 0.85 (0.42-1.71) | .650 |
| Acute abdomen | 1.9% (2) | 1.1% (7) | 1.74 (0.36-8.51) | .372 |
| Headache | 7.5% (8) | 7.2% (46) | 1.06 (0.48-2.31) | .888 |
| Hemoptysis | 2.8% (3) | 1.7% (11) | 1.67 (0.46-6.08) | .433 |
| Back pain | 13.2% (14) | 9.1% (58) | 1.53 (0.82-2.85) | .181 |
| Enlarged spleen | 3.8% (4) | 10.3% (66) | 0.34 (0.12-0.96) | .034 |
| Myalgia | 17% (18) | 13.7% (88) | 1.29 (0.74-2.24) | .371 |
| Arthralgia | 15.1% (16) | 16.2% (104) | 0.92 (0.52-1.63) | .769 |
| Confusion | 10.4% (11) | 12.5% (80) | 0.81 (0.42-1.58) | .543 |
| Meningitis | 1% (1) | 1.6% (10) | 0.61 (0.08-4.80) | >.999 |
| IS | ||||
| Hemorrhagic | 1.9% (2) | 3.4% (22) | 1.82 (0.42-7.86) | .528 |
| Ischemic | 14.2% (15) | 11.4% (73) | 0.80 (0.44-1.46) | .528 |
| Coma | 1.9% (2) | 2.8% (18) | 0.67 (0.15-2.92) | >.999 |
| Number of blood cultures, mean | 5.2±3.89 | 5.5±3.58 | 0.98 (0.93-1.03) | .425 |
95%CI, 95% confidence interval; BC, blood culture; COPD, chronic obstructive pulmonary disease; IS, ischemic stroke; OR, odds ratio.
Numbers in bold refer to P values of variables with statistically significant differences between the two groups.
Regarding the infection's entry site, local infections, intravenous catheters, and urinary infections were more frequent in group II (Table 1). Fever, shivering, enlarged spleen, and septic shock were more frequent in group II. In contrast, heart problems, particularly heart failure, were more frequent in BCN-E episodes. There were no statistical differences in the other forms of clinical presentation (Table 1).
To study the possible effect of antibiotic treatment on BCN-E episodes and to differentiate episodes of “true” BCN-E (no previous antibiotic treatment) from “amputated” BCN-E episodes (previous antibiotic treatment), the differences between BCN-E episodes with prior antibiotic use (n=36) and those without (n=54) were analyzed. Sixteen patients were excluded from the analysis because antibiotic use before blood extraction could not be determined. No statistically significant differences were found in most of the variables analyzed, except for a greater frequency of diabetes and community-acquired IE in the group of patients that had not received antibiotics. Fever prior to admission was more frequent in the group of patients with “amputated” BCN-E (Table 2, Table 3, Table 4).
Table 2. Demographic Variables, Predisposing Factors, Comorbidities, Entry Site, and Interval Between Symptom Onset and Diagnosis. Differences in the Group With Blood Culture-Negative Endocarditis Between Patients With and Without Antibiotic Treatment Prior to Blood Culture Extraction.
| Eepidemiological characteristics | BCN-E with prior antibiotics(n=36) | BCN-E without prior antibiotics (n=54) | OR (95%CI) | P |
| Age, years | 60±14 | 59±17.8 | 1 (0.98-1.03) | .805 |
| Symptoms until admission, days | 33.4±35.5 | 39.7±49 | 1 (0.98-1.01) | .661 |
| Origin | 47.2% (17) | 33.3% (18) | 1.79 (0.75-4.25) | .185 |
| Gender, males | 61.1% (22) | 66.7% (36) | 0.79 (0.33-1.89) | .590 |
| Community-acquired | 57.1% (20) | 79.2% (42) | 0.35 (0.14-0.90) | .026 |
| Nosocomial | 31.4% (11) | 20.4% (11) | 2.86 (1-7.14) | .266 |
| Previous heart disease | 69.4% (25) | 64.8% (35) | 1.23 (0.50-3.04) | .648 |
| Comorbidities | ||||
| Diabetes | 5.6% (2) | 24.1% (13) | 0.19 (0.04-0.88) | .021 |
| Chronic renal failure | 13.9% (5) | 9.3% (5) | 1.58 (0.42-5.91) | .513 |
| Dialysis | 16.7% (2) | 0% (0) | Undetermined | .077 |
| Cancer | 8.3% (3) | 7.4% (4) | 1.14 (0.24-5.41) | >.999 |
| COPD | 8.3% (3) | 7.4% (4) | 1.14 (0.24-5.41) | >.999 |
| Immunosuppression | 8.3% (3) | 5.6% (3) | 1.54 (0.29-8.12) | .680 |
| Entry site | ||||
| Local infection | 11.1% (4) | 3.7% (2) | 3.25 (0.36-20) | .213 |
| Previous surgery | 11.1% (4) | 9.3% (5) | 1.22 (0.30-5) | >.999 |
| Dental manipulation | 11.1% (4) | 13% (7) | 0.84 (0.23-3.12) | >.999 |
| Clinical presentation | ||||
| Fever | 77.8% (28) | 66.7% (36) | 1.75 (0.6-4.61) | .255 |
| Cardiologic | 52.8% (19) | 55.6% (30) | 1.89 (0.38-2.08) | .795 |
| Neurologic | 8.3% (3) | 20.4% (11) | 0.35 (0.09-1.38) | .123 |
| Renal | 8.3% (3) | 5.6% (3) | 1.54 (0.29-8.12) | .680 |
| Constitutional syndrome | 37.1% (13) | 24.1% (13) | 1.86 (0.74-4.71) | .185 |
| Signs/symptoms upon admission | ||||
| New murmur | 44.4% (16) | 46.3% (25) | 0.93 (0.40-2.17) | .863 |
| Shivers | 40.7% (11) | 40% (20) | 1.03 (0.40-2.68) | .950 |
| Heart failure | 54.3% (19) | 44.4% (24) | 1.48 (0.63-3.49) | .364 |
| Fever upon admission | 69.4% (25) | 51.9% (28) | 2.11 (0.87-5.13) | .097 |
| Fever prior to admission | 78.8% (26) | 54% (27) | 3.16 (1.16-8.63) | .021 |
| Renal failure | 22.2% (8) | 13% (7) | 1.92 (0.63-5.86) | .248 |
| Septic shock | 0% (0) | 1.9% (1) | Undetermined | >.999 |
| Back pain | 16.7% (6) | 14.8% (8) | 1.15 (0.36-3.65) | .812 |
| Enlarged spleen | 8.3% (3) | 1.9% (1) | 4.73 (0.47-47.4) | .299 |
| Myalgia | 25% (9) | 16.7% (9) | 1.67 (0.60-4.71) | .333 |
| Arthralgia | 19.4% (7) | 13% (7) | 1.62 (0.52-5.10) | .406 |
| Confusion | 2.8% (1) | 14.8% (8) | 0.16 (0.02-1.37) | .080 |
| Meningitis | 0% (0) | 1.9% (1) | Undetermined | >.999 |
| IS | 8.3% (3) | 18.5% (10) | 0.39 (0.10-1.54) | .217 |
95%CI, 95% confidence interval; BCN-E, blood culture-negative endocarditis; COPD, chronic obstructive pulmonary disease; IS, ischemic stroke; OR, odds ratio.
Numbers in bold refer to P values of variables with statistically significant differences between the two groups.
Table 3. Echocardiographic Findings and Clinical Course. Differences in the Group With Blood Culture-Negative Endocarditis Between Patients With and Without Antibiotic Treatment Prior to Blood Culture Extraction.
| Findings | BCN-E with prior antibiotics(n=36) | BCN-E without prior antibiotics(n=54) | OR (95%CI) | P |
| Damaged valve | ||||
| Native | 57.1% (20) | 63% (34) | Reference | .592 |
| Prosthetic | ||||
| Metallic | 31.4% (11) | 31.5% (17) | 1.10 (0.43-2.81) | |
| Biological | 11.4% (4) | 5.6% (3) | 2.26 (0.46-11.19) | |
| Valve failure ≥ moderate | 75% (27) | 74.1% (40) | 1.05 (0.40-2.77) | .921 |
| Periannular complications | 33.3% (12) | 33.3% (18) | 1 (0.41-2.45) | >.999 |
| Vegetation (TEE) | 72.2% (26) | 77.8% (42) | 0.74 (0.28-1.96) | .548 |
| Size of vegetation (mm), average | 14.7 (7.22) | 14.6 (12.5) | 0.96 (0.41-2.27) | .930 |
| Clinical course | ||||
| Systemic embolism, CNS | 8.3% (3) | 27.8% (15) | 0.24 (0.06-0.89) | .024 |
| Renal failure | 13.9% (5) | 18.5% (10) | 0.71 (0.22-2.28) | .058 |
| Septic shock | 5.9% (1) | 14.3% (2) | 0.37 (0.03-4.63) | .576 |
| Heart failure at any time | 68.4% (13) | 50% (9) | 2.17 (0.57-8.25) | .254 |
| Persistent infection | 15.8% (3) | 17.2% (5) | 0.90 (0.19-4.30) | >.999 |
| Surgery | 55.6% (20) | 55.6% (30) | 1 (0.43-2.34) | >.999 |
| Death | 30.6% (11) | 24.1% (13) | 1.39 (0.54-3.57) | .496 |
95%CI, 95% confidence interval; BCN-E, blood culture-negetive endocarditis; CNS, central nervous system; OR, odds ratio; TEE, transesophageal echocardiogram.
Numbers in bold refer to P values of variables with statistically significant differences between the two groups.
Table 4. Analysis of the Main Diagnostic and Prognostic Variables, Excluding Cases of Possible Infective Endocarditis, According to the Criteria of Li et al. 19
| Variables | Negative BC(n=80)Definitive IE | Positive BC(n=643) | OR (95%CI) | P |
| Previous antibiotic treatment | 35.4% (28) | 30% (192) | 1.36 (0.81-2.29) | .634 |
| Symptoms until admission, days | 31.8 (38.9) | 41.5 (80.1) | 1 (0.99-1) | .252 |
| Signs and symptoms until admission | ||||
| Heart failure | 44.3% (35) | 38% (243) | 1.30 (0.81-2.08) | .275 |
| Fever prior to admission | 59.5% (44) | 72.1% (430) | 0.57 (0.34-0.93) | .024 |
| Fever upon admission | 60% (48) | 73.3% (469) | 0.55 (0.34-0.88) | .013 |
| Renal failure | 18.8% (15) | 15.3% (98) | 1.28 (0.70-2.34) | .419 |
| Echocardiographic findings | ||||
| Vegetations | 75% (60) | 83.7% (538) | 0.59 (0.34-1.01) | .053 |
| Periannular complications | 37.5% (30) | 33.1% (213) | 1.21 (0.75-1.96) | .435 |
| Clinical course | ||||
| Heart failure | 56.3% (18) | 52.1% (139) | 1.18 (0.57-2.48) | .654 |
| Renal failure | 10% (8) | 19.8% (127) | 0.45 (0.21-0.96) | .035 |
| Persistent infection | 18.8% (9) | 29.8% (105) | 0.54 (0.25-1.16) | .127 |
| Surgery | 61.3% (49) | 55.5% (357) | 1.27 (0.79-2.04) | .330 |
| Death | 26.3% (21) | 30.8% (198) | 0.80 (0.47-1.35) | .404 |
95%CI, 95% confidence interval; BC, blood culture; IE, infective endocarditis; OR, odds ratio
Numbers in bold refer to P values of variables with statistically significant differences between the two groups.
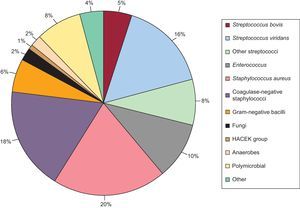
During hospitalization, the microorganism causing IE was identified in 21 episodes (19.8%) in group I. In most cases, the pathogen was isolated from surgical specimens. The effectiveness of the serological tests was low; Q fever accounted for 2% of cases and Brucella for 1%. The microbiological profile of the episodes in group II is shown in Figure 1. There were no differences in the mean number of blood cultures drawn between the 2 groups (Table 1).
Figure 1. Microbiological profile of infective endocarditis episodes in group II.
The subanalyses performed to study the time trend of the microbiological profile in IE in our cohort showed no differences in the distribution of BCN-E and BCP-E over time (period 1: BCN-E 12.4%, BCP-E 87.6%; period 2: BCN-E 15.4%, BCP-E 84.6%; period 3: BCN-E 15%, BCP-E 85%; P=.599). The microbiological profile of the BCP-E group showed no differences between the 3 periods, with the exception of 3 groups of microorganisms (coagulase-negative staphylococci, fungi and other non-viridans, non-bovis streptococci) (Table 5).
Table 5. Temporal Evolution of the Microbacteriological Profile of Infective Endocarditis.
| Microorganism | Period 1(n=217) | Period 2(n=181) | Period 3(n=172) | P |
| Streptococcus bovis | 10 (4.6%) | 9 (5%) | 11 (6.4%) | .799 |
| Streptococcus viridans | 40 (18.4%) | 30 (16.6%) | 24 (13.9%) | .508 |
| Enterococcus | 23 (10.6%) | 13 (7.2%) | 22 (12.8%) | .212 |
| Other streptococcus | 23 (10.6%) | 7 (3.9%) | 15 (8.7%) | .042 |
| Staphylococcus aureus | 40 (18.4%) | 39 (21.5%) | 32 (18.6%) | .685 |
| Coagulase-negative staphylococcus | 35 (16.1%) | 44 (24.3%) | 24 (14%) | .026 |
| Gram-negative bacilli | 13 (6%) | 12 (6.6%) | 7 (4.1%) | .555 |
| Fungi | 6 (2.8%) | 0 (0%) | 7 (4.1%) | .031 |
| HACEK group | 0 (0%) | 3 (1.7%) | 1 (0.6%) | .138 |
| Anaerobes | 3 (1.4%) | 5 (2.8%) | 4 (2.3%) | .611 |
| Polymicrobial | 16 (7.4%) | 16 (8.8%) | 16 (9.3%) | .761 |
| Other | 8 (3.7%) | 3 (1.7%) | 9 (5.2%) | .186 |
Numbers in bold refer to P values of variables with statistically significant differences between the two groups.
The presence of vegetations detected by TEE was less frequent in group I (72.6% vs 83.8%; P=.005). There were no significant differences between the 2 groups in the percentage of periannular complications or in the presence and severity of valve failure. There were no differences in the localization of the infection (Table 6).
Table 6. Echocardiographic Findings.
| Findings | Negative BC(n=106) | Positive BC(n=643) | OR (95%CI) | P |
| Damaged valve | ||||
| Native | 56.2% (59) | 61.3% (387) | Reference | .606 |
| Prosthetic | 44.3% (47) | 39.2% (251) | .313 | |
| Metallic | 34.3% (36) | 30.4% (192) | 0.83 (0.53-1.29) | .606 |
| Biological | 9.5% (10) | 8.2% (52) | 0.82 (0.40-1.70) | .606 |
| LVEF | ||||
| Normal | 79.5% (66) | 82.6% (403) | Reference | .213 |
| Mildly affected | 6% (5) | 9.4% (46) | 1.51 (0.58-3.94) | |
| Moderately affected | 9.6% (8) | 5.7% (28) | 0.58 (0.25-1.32) | |
| Severely affected | 4.8% (4) | 2.3% (11) | 0.45 (0.14-1.46) | |
| Valve failure ≥ moderate | 69.8% (74) | 68.1% (438) | 1.08 (0.69-1.69) | .728 |
| Periannular complications | 32.1% (34) | 32.8% (211) | 0.97 (0.62-1.50) | .880 |
| Abscesses | 21.7% (23) | 21% (135) | 1.04 (0.63-1.72) | .869 |
| Pseudoaneurysms | 14.2% (15) | 17.6% (113) | 0.77 (0.43-1.38) | .386 |
| Fistula | 3.8% (4) | 4.2% (27) | 0.89 (0.31-2.61) | >.999 |
| Vegetation (TEE) | 72.6% (77) | 83.8% (539) | 0.51 (0.32-0.82) | .005 |
| Size of vegetation (mm), mean | 15.4 (10.9) | 13.5 (7.2) | 0.87 (0.54-1.40) | .575 |
95%CI, 95% confidence interval; BC, blood culture; LVEF, left ventricular ejection fraction; OR, odds ratio; TEE, transesophageal echocardiogram.
Numbers in bold refer to P values of variables with statistically significant differences between the two groups.
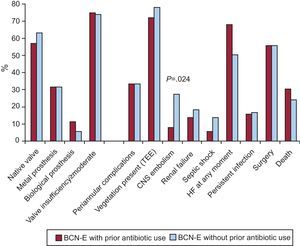
There were no differences in any of the electrocardiographic parameters analyzed among patients with BCN-E, irrespective of whether they received antibiotic treatment before blood culture extraction (Figure 2, Table 3).
Figure 2. Echocardiographic findings and clinical course. Differences in the blood culture-negative group between patients with and without antibiotic treatment prior to blood culture extraction. BCN-E, blood culture-negative endocarditis; CNS, central nervous system; HF, heart failure; TEE, transesophageal echocardiogram.
Clinical CourseDuring hospitalization, the clinical course was similar in both groups. There were no significant differences in the development of heart failure, renal failure, the presence of signs of persistent infection or septic shock (Table 7). The percentage of embolisms in the central nervous system was similar in both groups, but the number of embolisms in the liver-kidney axis and spleen was higher in episodes in group II. A similar number of patients underwent surgery in the 2 groups, the most frequent cause being heart failure. There were no differences in mortality or its causes between the 2 study groups.
Table 7. Clinical Course.
| Course until surgery | Negative BC(n=106) | Positive BC(n=643) | OR (95%CI) | P |
| Systemic embolism | ||||
| CNS | 19.8% (21) | 20.5% (132) | 0.96 (0.57-1.6) | .865 |
| Liver-renal axis | 1.9% (2) | 10.7% (69) | 0.16 (0.04-0.66) | .004 |
| Spleen | 1.9% (2) | 10.3% (66) | 0.17 (0.04-0.70) | .005 |
| Liver | 0% (0) | 0.8% (5) | Undetermined | >.999 |
| Kidney | 0% (0) | 2.3% (15) | Undetermined | .147 |
| Renal failure | 17% (18) | 19.6% (126) | 0.84 (0.49-1.44) | .527 |
| Death | 25.5% (27) | 30.6% (197) | 0.77 (0.48-1.24) | .282 |
| Surgery | 57.5% (61) | 55.5% (357) | 1.09 (0.72-1.64) | .697 |
| Septic shock | 8.6% (3) | 8.5% (21) | 1.00 (0.28-3.56) | >.999 |
| Heart failure at any time | 57.1% (24) | 52.1% (139) | 1.23 (0.64-2.37) | .540 |
| Persistent infection | 20% (6/30) | 23.9% (50) | 0.79 (0.31-2.05) | .635 |
95%CI, 95% confidence interval; BC, blood culture; CNS, central nervous system; OR, odds ratio.
Numbers in bold refer to P values of variables with statistically significant differences between the two groups.
To avoid any bias in the results of the study, the analysis was repeated after withdrawing the episodes diagnosed as “possible” BCN-E. In this subanalysis the results of the clinical course and the prognosis were, once again, similar (Table 4).
Within the BCN-E group, a greater frequency of embolisms in the central nervous system were observed in the group of patients that had not received antibiotic treatment prior to blood culture extraction. A similar number of patients underwent surgery in both groups and there were no differences in mortality (Figure 2).
DiscussionThe aim of our study was to determine the present situation of BCN-E and its prognosis.
BCN-E accounts for 2.5%-31% of the total number of IE episodes, depending on the series.24, 25 In our cohort, the prevalence of BCN-E episodes was 14%. There are 3 reasons for blood culture-negative in patients with left IE: the causative microorganism has slow growth (Propionibacterium acnes, etc.) or requires special cultivation methods (Coxiella, Bartonella, Tropheryma whipplei, etc.); fungal endocarditis (Aspergillus, etc.); and the administration of antibiotics in the days prior to blood culture extraction. Many authors report that the latter is the most significant cause of BCN-E.5, 7, 8, 9, 10, 11, 12 In some series, the percentage of patients with BCN-E that received antibiotic treatment 1 week before blood culture extraction varied between 61.9% and 66%.8, 9 In our series, this percentage was much lower and there were no differences in the prior administration of antibiotics between the BCN-E and the BCP-E groups (34.3% vs 30%, P=.812). There are 2 explanations for these notable differences: greater knowledge of IE in outpatient care and a lower frequency of physicians prescribing antibiotics without having first requested blood cultures, and a change in the microbacteriological profile of the disease. Currently, S aureus is the most frequent microorganism in IE, ahead of staphylococci, which classically occupied that place.13, 26 The former often grows in blood cultures despite prior antibiotic treatment; however, microorganisms of low and medium virulence are highly susceptible to bactericidal antibiotics and blood cultures are often negative after antibiotic administration. In our series, antibiotics had been administered before blood culture extraction in 29.9% of the episodes testing positive for S aureus.
In contrast, some authors maintain that the amount of blood and, correspondingly, the number of blood cultures collected could influence and increase the probability of isolating the causative agent,27 especially in cases caused by microorganisms of low virulence. In our cohort there was no difference in the mean number of blood cultures extracted between the 2 groups.
Unlike other historical series, we performed TEE in all patients, which undoubtedly contributed to diagnosis of the disease. Importantly, as reported in other studies,9 clinical judgement had high sensitivity in the diagnosis of BCN-E. The Duke criteria have been shown to have high sensitivity and specificity in the diagnosis of IE.28 However, in some situations, such as BCN-E, these criteria lose diagnostic accuracy and consequently several modifications have been proposed which increase their sensitivity without losing specificity.19 Kupferwasser et al. observed that in comparison with the Duke criteria, the exclusively clinical criterion showed the same sensitivity for the diagnosis of BCN-E, but with lower specificity.29
As regards the delay in IE diagnosis, the data from our series showed that the interval between symptom onset and diagnosis was similar in both groups (a mean of 34 days in group I and 42 days in group II; P=.544). Furthermore, the subanalysis in the BCN-E group also showed that the delay in diagnosis was no greater in the group of patients which had received previous antibiotic treatment. Importantly, the patients included in our database live in urban health areas, with easy access to health services, which involves a shorter delay in the demand for health care and in performing examinations and complementary tests (transthoracic echocardiogram), leading to more timely diagnosis.
The lower frequency of fever, both before and during admission, shivering, and septic shock in the BCN-E populations suggests that there is a greater ratio of low virulence microorganisms causing IE. However, this lower frequency of infectious manifestations is not accompanied by less valve damage as there was a greater frequency of heart problems. Possibly, diagnosis of IE in these patients is based more on cardiologic manifestations than on infectious symptoms, requiring the performance of an echocardiogram, which, given the availability and sensitivity of this technique, avoids diagnostic delay.
In this study, TEE detected vegetations at a lower frequency in the BCN-E group than in the BCP-E group. Other authors have obtained similar results.30 Antibiotic administration before blood culture extraction in some cases of BCN-E prevents the growth of the microorganism causing IE, and, furthermore, could also “amputate” the appearance or decrease the size of the maximum echocardiographic expression of the disease, namely, vegetations. In addition, some of the microorganisms which cause BCN-E, such as Propionibacterium acnes or Coxiella burnetii, have fewer vegetations and these are smaller.31, 32 All of these considerations would lead to a lower detection of vegetations in this group of patients with BCN-E. In our study, there were no significant differences in the detection of vegetations or their size between the 2 BCN-E groups (with or without antibiotic treatment). Thus, there may be other reasons that explain why there are fewer vegetations in BCN-E episodes.
Unlike prior publications1, 2, 3, 4, 5 in which BCN-E was associated with a higher rate of periannular complications,4 a greater degree of heart failure, greater need for surgery,3, 4, 5, 12 and higher intrahospital mortality in the long term,1, 2, 3 in our study the percentage of adverse clinical events was similar in both groups, irrespective of the blood culture results. Equally, there were no differences in the proportion of patients undergoing surgery or in mortality rates. These results were repeated in the subanalyses that excluded possible IE cases within the BCN-E group and coincide with those of several recent series.12, 32, 33 The findings that there was no delay in the diagnosis and treatment of BCN-E patients and that this type of IE was associated with less virulent microorganisms–both in “true” cases of BCN-E (caused by microorganisms with a limited growth capacity in conventional blood cultures), and, in “decapitated” IE (due to the prior use of antibiotics and to the fact that the causative agent was most likely a streptococcus or a coagulase-negative staphylococcus)–explain why today BCN-E does not have a worse prognosis than BCP-E.
To study the temporal evolution of IE in our series, and to analyze the possible effect of the time factor on the microbiological profile, we performed a subanalysis in which the study period was divided into 3 similar intervals (Table 5). There were no significant differences in the microbiological profile over time or in the percentage of methicillin-resistant microorganisms. Differences were observed in only 3 groups of microorganisms: coagulase-negative staphylococci, fungi, and other non-viridans, non-bovis streptococci. None of these 3 groups showed a time tendency, and all were heterogeneous groups that included many types of microorganisms with different behavior and virulence. With the exception of coagulase-negative staphylococci, in the other 2 groups in which differences were found, the number of cases was too small to allow conclusions to be drawn.
Despite the known diagnostic significance of S aureus, there were no differences in the number of cases caused by this microorganism among the 3 time intervals. Furthermore, based on the above-mentioned results in our cohort, the time factor did not play a significant role in the behavior and diagnosis of the disease.
In the BCN-E group, there were no differences in epidemiological variables or in predisposing factors for the development of IE, except for a greater presence of fever prior to admission in the group receiving antibiotic treatment before blood culture extraction. This finding is unsurprising, since fever is a frequent reason for empirical antibiotic therapy without blood culture extraction in outpatient care and for self-medication by patients. As to the clinical course within this group, central nervous system embolisms were more frequent in the group not receiving antibiotic treatment before blood culture extraction, which could be explained by the effect of antibiotics in reducing embolic risk, as reported in prior studies.34
In contrast, and contrary to earlier publications,35 there were no differences in mortality or in the need for surgery within the BCN-E group, irrespective of antibiotic use before blood culture extraction.
LimitationsAlthough patients were included prospectively and consecutively, this study was retrospective, which implies certain bias. Our hospitals are reference centers for surgery and many patients have been referred to us for surgery. As a result, our patients may constitute a select group, with more severe IE.
In both groups, the length of antibiotic treatment before blood culture extraction was unknown. Similarly, in BCP-E, there was no record of whether or not the causative microorganism responded to the previous antibiotic therapy. These 2 factors probably influenced the results of the blood cultures.
The number of patients in the 2 groups differed, which may have influenced the statistical analysis; however, the overall sample size is large and the impact of this difference on the results would be minimal.
Molecular amplification techniques in blood or tissue from surgery were used anecdotally in this series, as these procedures are very recent. If they had been used systematically, the causative agent might have been identified in a greater number of BCN-E episodes. We did not employ immunoblotting methods using specific antibacterial antibodies or other more recent techniques, which could have increased our ability to identify the causative agent. However, the results of other studies which used sophisticated valve tissue immunohistochemistry and molecular biological methods reveal that these techniques were not highly sensitive.36
ConclusionsCurrently, previous antibiotic administration is not more frequent in patients with BCN-E. This entity does not involve a delay in the diagnosis or in the start of appropriate treatment compared with BCP-E cases. The results for intrahospital clinical course, need for surgery, and prognosis are similar in both BCN-E and BCP-E.
Conflicts of interestNone declared.
Received 3 February 2012
Accepted 10 April 2012
Corresponding author: Instituto Cardiovascular, Hospital Clínico San Carlos, Prof. Martín Lagos s/n, 28040 Madrid, Spain. carlosferreraduran@gmail.com